1. INTRODUCTION, HISTORICAL BACKGROUND, AND CURRENT CONTEXT FOR THE SEARCH FOR BIOSIGNATURES
The carbon cycle is a complex series of processes that governs the interactions between the biosphere and the geosphere and has changed significantly since the formation of the Earth ~4.5 billion years ago (Gya; Des Marais, 2001). Many of these differences reflect how distinct the Archean world was from the modern one: The mantle was hotter, the partial pressure of atmospheric CO2 was higher (perhaps as high as 1 bar), and there was little to no O2 in the atmosphere and oceans (Schopf & Klein, 1992). The biosphere was nascent and early in its evolutionary development (reviewed by Schopf, 1983). Prior to the evolution of oxygenic photosynthesis and the Great Oxygenation Event (GOE), it is thought that the productivity of the biosphere was limited (reviewed by Hayes et al., 1983; Schidlowski, 2001) and thus the flux of carbon between atmospheric CO2 and organic carbon would have been lower than that flux in the modern era (Des Marais, 2001). The GOE introduced a major shift in the global carbon cycle and a tremendous increase in global productivity (reviewed by Fischer et al., 2016), which has been debated as being associated with either an increase or a decrease in the relative rates of organic carbon burial over time (for example, Catling et al., 2001; Des Marais, 2001; Krissansen-Totton et al., 2015; Marais et al., 1992; Schidlowski, 1988, among others).
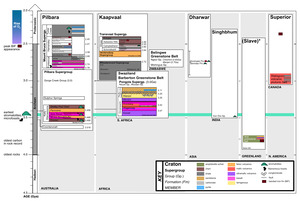
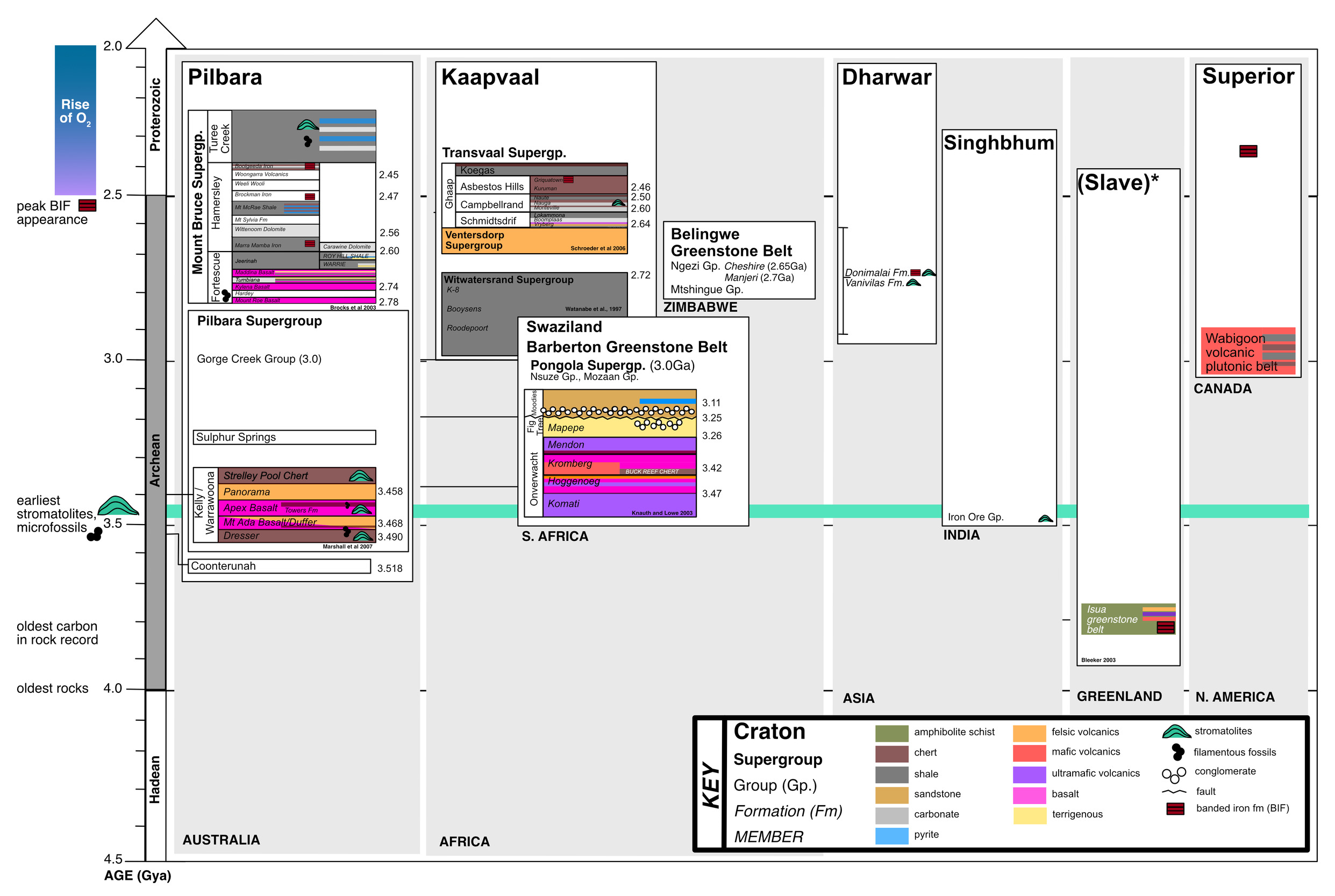
The geological record prior to the GOE is limited due to Earth’s tectonics and rock cycle. Indeed, only 35 Archean cratons have been identified (fig. 1; Bleeker, 2003; Strauss & Moore, 1992), and these are thought to be derived from an even smaller number of super-cratons (that is, 3-5; Bleeker, 2003). The most well-studied formations containing Archean organic carbon are located in Western Australia, Greenland and South Africa. However, as far as we currently understand, kerogen is the only phase of organic matter that remains from the Archean. The interpretation of this refractory carbon has been a long-standing question for scientists interested in understanding putative biosignatures found in the oldest rocks on Earth.
Kerogen is a macromolecular carbon-rich material, insoluble in acid, base, or organic solvent, and thus operationally defined because it represents what is left when the solvent extractable organic compounds (bitumen) are removed. It is thought to be predominantly made up of degradation products of cell membranes, carotenoids, chlorophylls, wax esters, triglycerides, cross-linked proteins, and resins, and classified into sub-types based on elemental ratios (that is, H/C and O/C), mineral associations, and whether the original biomolecular components are thought to have a marine, lacustrine or terrestrial origin (see Vandenbroucke & Largeau, 2007 for a review on prior studies of kerogen).
Because minimal molecular information is retained after biomass degrades to become kerogen, much of this debate focuses on interpretations of its carbon isotopic composition (for example, Craig, 1954; Horita, 2005; Mojzsis et al., 1996; Rankama, 1954). Most of these studies operate under the premise that carbon isotope values are relatively unsusceptible to major shifts through the post-depositional processes that affect organic carbon in the rock cycle, and thus could provide clues into the metabolisms or abiogenic reactions that were dominant on early Earth. Indeed, in 1954, Kalervo Rankama argued that carbon isotope values substantially lower than the isotopic composition of the bulk-Earth—similar to the carbon isotopic composition of modern biomass—in Archean units must be evidence of life and thus can be used to interpret the Archean carbon isotope record as definitive evidence of early life on Earth (Rankama, 1954). Soon after, Harmon Craig challenged this uniformitarian argument, suggesting that the low carbon isotope values of refractory carbon do not necessarily require biology: These arguments questioned the use of low carbon isotope values to invoke biogenicity and highlighted that such assumptions underlie “unsolved problems in the geochemistry of carbon which must be investigated” (Craig, 1954).
Since the early Precambrian (meta-)sedimentary rock record was first described, much of the research on the carbon isotopic composition of the kerogen it contains has aimed to characterize the early biosphere through focused studies on specific localities, lithologies, and time frames (for example, Brocks, Summons, et al., 2003; Eigenbrode & Freeman, 2006; French et al., 2015; Slotznick & Fischer, 2016; Ueno et al., 2002). Among these localities, some putative microfossils exist (see Supplementary Text for additional details on “Studies of Archean biomarkers, microfossils, and associated lithology”). In other instances, organic matter is nearly graphitic in its composition, but light carbon isotope values have been measured and have been proposed to be of a biological origin, for instance, in carbonaceous mineral inclusions from Isua and Akilia (3.8Gya) (Mojzsis et al., 1996) and other early Archean mineral inclusions (McKeegan et al., 2007; Papineau et al., 2011).
These Isua and Akilia studies were valuable in the context of origins of life research. If the inclusions derived from a source rock that was of sedimentary origin, the depositional environment of the original units containing these inclusions could plausibly be interpreted as a submarine/deep marine environment whose ultramafic lithologies hint at the remnants of an Archean hydrothermal vent system. However, it is debated whether the Isua and Akilia protoliths are of sedimentary origin. Contrary to initial interpretations, it has been suggested the organic matter (OM) found within these apatites could potentially derive from either an igneous or other abiotic source (Fedo, 2000; Nutman et al., 1984). At least in principle, the light carbon isotope values of these materials can also be explained by Rayleigh distillation loss of CO2 from partial decomposition of abiogenic carbon (Eiler et al., 1997). For even older and more altered samples, measurements of carbon isotope values of indigenous organic carbon are challenging. Many studies have measured and reported δ13C values of graphite inclusions found within Hadean zircons, but only one measurement remains unchallenged as primary carbon rather than contamination (Bell et al., 2015).
The carbon isotopic composition of refractory carbon still represents an “unsolved problem in the geochemistry of carbon” (Craig, 1954). Prior studies have worked to address this problem by compiling datasets of measurements of the carbon isotope contents of Archean samples, and performing statistical analyses on them to identify any trends that could link the data to biology (Des Marais, 2001; Hayes et al., 1983; Krissansen-Totton et al., 2015; Lepot, 2020; Strauss & Moore, 1992). Our study aims to re-address this long-standing question and was inspired by recent and current missions to collect and return samples from extraterrestrial bodies, where macromolecular organic matter is anticipated to be the dominant form of preserved carbon, requiring that any interpretations of isotopic composition of such materials are well-understood. First, we assembled the largest compilation to-date of carbon isotope ratios measured from Archean samples. Along with this compilation, we assembled descriptions of the mineralogy, lithology, and metamorphic grade associated with each Archean sample. Notably, we identified a bimodality within the Archean carbon isotope data that has not been found in prior data compilations, which was observed even with subsampling to account for geographic and stratigraphic biases. To interpret these results, we synthesized existing constraints on the evolution of organic carbonaceous matter through the rock cycle to construct an empirical framework and a model for the change in the carbon isotopic composition of organic matter from deposition through high-grade metamorphism. Finally, we applied this model to re-interpret particularly anomalous Archean organic carbon isotope records and to propose next steps for studies in this field.
2. A REVIEW OF PUBLISHED CONSTRAINTS ON THE ARCHEAN CARBON ISOTOPE RECORD
2.1. Carbon isotope nomenclature, measurements and methods
We follow common nomenclature by quantifying carbon isotope ratios using “delta” notation, which reports the contrast between the 13C/12C ratio, also referred to as so-called “R values,” through the formula:
δ13C=(13Rsample13Rstandard−1),
where 13R is the ratio of 13C/12C in a sample or standard as denoted in the formula by the subscript. All data presented here are reported relative to the Vienna Peedee Belemnite carbon isotope standard (VPDB) and are reported in units of per-mille (‰). Any subscripts included with delta values serve as descriptors for the substrate, such as total organic carbon (TOC) or kerogen. We distinguished TOC measurements from those of specifically targeted and extracted kerogen, which are prepared by removing all minerals and soluble organics from samples. In our data compilation, we did not average any measurements for given localities and instead present reported measurements for each individual replicate that exists within the literature. When referring to trends observed in the carbon isotopic composition of Archean organic carbon for both TOC and kerogen, we refer to data as δ13Corg values. Our data compilation included carbon isotope ratios measured with a range of geochemical methods, but most of the measurements were made by combusting samples to convert organic carbon to CO2, with subsequent isotope ratio analysis. Such analyses have been mostly performed in recent years with an Elemental Analyzer, followed by isotopic analysis of the produced CO2 using a gas source isotope ratio mass spectrometer.
We compiled δ13Corg values along with metadata for the geologic localities, dominant lithology of the units, % TOC (when available), and two different proxies for thermal maturity. Thermal maturity is also sometimes characterized via RockEval, which measures, among other properties, the H/C ratio: The H/C ratio decreases as metamorphic grade increases (see sections 2 and 3). We use both H/C ratios and qualitative descriptions of metamorphic grade based on petrographic observations to interpret the effect of metamorphism on δ13Corg values. The full data compilation, including the original source of the data, locality, and lithology information, available % TOC, H/C ratios and metamorphic grade are included in Zeichner, 2023. Data reduction and statistical analyses for this study and plots were performed and generated in RStudio (RStudio team, 2020).
2.2. Pre-GOE carbon isotope values and interpretation
2.2.1. Total organic carbon and kerogen carbon isotope values
δ13Corg values of Archean TOC (n=2421) and kerogen (n=556) are plotted versus time in figure 2A. To add context to our compilation and facilitate interpretation, we plotted our data alongside a previous compilation of δ13Ccarbonate values of Archean carbonate-bearing rocks (n=731, fig. 2A; where each data point may represent averages across several replicate measurements; Krissansen-Totton et al., 2015). Archean TOC has a mean δ13CTOC value of −30.5±0.16 (1 standard error (SE); the standard deviation (SD) of individual measurements is ±8.0‰) and a median value of −30.7‰; isolated kerogen from Archean rocks has a mean δ13Ckerogen value of −33.7±0.48 (1 SE; the SD of individual measurements is ±11.3‰) and a median value of −33.65‰. These populations are statistically significantly different from one another based on two-sample Kolmogorov-Smirnov test (p-value < For further comparison, analysis of a prior compilation of Phanerozoic δ13CTOC values gave a mean δ13CTOC value of −26.7±0.22 (1 SE for n = 449; the SD of individual measurements is ±4.6‰) and a median δ13CTOC value of −27‰ (where each data point may represent averages across several replicate measurement; Krissansen-Totton et al., 2015). Both the TOC and the kerogen carbon isotopic compositions of Archean samples compiled here were statistically significantly different from the carbon isotopic composition of the Phanerozoic TOC samples, based on two-sample Kolmogorov-Smirnov test (p-values < Together, these results demonstrated that the carbon isotopic composition of sedimentary rocks deposited in Archean basins is systematically and significantly lower than similar deposits generated over the past ~500 million years, which supports results of prior data compilations (for example, Des Marais, 2001; Hayes et al., 1983; Krissansen-Totton et al., 2015; Lepot, 2020; Strauss & Moore, 1992), but includes an order of magnitude more data than in the first compilation of this record (Hayes et al., 1983).
To explore the differences between the δ13Corg values of Archean TOC and Archean kerogen, as well as the difference between the isotopic compositions of both of these populations with that of Phanerozoic organic carbon, we plotted our data as smoothed histograms, or so-called ‘kernel density estimates’ (fig. 2B). δ13Corg values of Archean OM are more variable than those of the Phanerozoic, which is reflected by our calculated SDs (reported above) as well as the kernel density estimates (fig. 2B). While the average δ13Corg value of Archean OM is lower than the average δ13Corg value of Phanerozoic OM, the Archean values span a wide range from values as low as ~ −60‰ up to values as high as 1.8‰. Outliers within the data set are concentrated within specific time frames and correspond to specific localities/formations. For instance, high δ13Corg values of −15 to 1.8‰ were found within ~3.8Gya rocks from Isua and Akilia, while low δ13C values of −40 to −60‰ were found within rocks from the Fortescue, Hamersley, and Superior Groups (note: the well-studied Tumbiana formation is part of the Fortescue group).
The distribution of the Archean δ13CTOC values is approximately unimodal (based on Hartigan’s dip test; D = 0.007625, p-value = 0.4938), whereas the distribution of the Archean δ13Ckerogen values appears to be bimodal (based on Hartigan’s dip test; D = 0.040431, p-value = To our knowledge, this bimodality has not been identified by prior studies of the Archean carbon isotope record. We subsampled the data to test whether multimodality within the kernel density estimate of distributions of δ13Ckerogen was an artifact of preferential sampling (that is, whether specific localities known to have OM with anomalous isotopic compositions were sampled more; fig. 3). Multimodality appears to be preserved even with subsampling which suggests that the spread in values is recording a real feature of the record (fig. 3). This interpretation was supported by focused studies of specific formations from the Pilbara craton. We found that multimodal features with maxima at both higher (−10‰) and lower (−45‰) δ13Ckerogen values are preserved within samples with different dominant lithologies (fig. 3 & Supplemental fig. 3), which further suggested that the bimodality is not isolated to a single lithology or even a single formation but rather a feature that is recorded across many formations within a craton within an interval in geologic time. These variations in the record of δ13Corg values inspire a need to understand the potential factors—both biological and post-depositional—that can cause such variation.
2.2.2. Differences in carbon isotope values by lithology
It has been of longstanding interest to use variations in Archean δ13Corg values as a proxy for and evidence of microbial ecology, which might vary based on depositional environments and their potential to support microbes that use distinct carbon fixation pathways (Des Marais, 2001; Eigenbrode & Freeman, 2006). Such patterns may be reflected in the systematic variation in δ13Corg values by lithology or formation that are not visible when the data is sorted by eon (Supplemental fig. 1). Some studies have demonstrated that shale-hosted OM has lower δ13Corg values than carbonate hosted OM for samples in both the Pilbara and Kaapvaal cratons (Eigenbrode & Freeman, 2006; Fischer et al., 2009; Strauss & Beukes, 1996). Using our dataset, we reanalyzed prior measurements of δ13Corg from units that date to ~2.7 Ga that did not experience high temperature metamorphism (that is, these samples are limited to sub-greenschist facies peak conditions) to probe the relationship between δ13Corg values, lithology, and depositional environment (Supplemental fig. 2C). Based on kernel density estimates of distributions of the δ13Corg values from samples from the Hamersley basin, Pilbara Craton, carbonate, and chert hosted OM had higher carbon isotope values than the values for organic phases hosted within other lithologies (including shale; Supplemental fig. 2C). For both the Hamersley and Fortescue groups, chert-hosted OM had higher δ13Corg values than OM hosted in other lithologies (Supplemental fig. 2B&C).
These higher values in chert-hosted OM may be driven by the fact that carbonate and chert are often co-associated, and that chert-associated samples often have low TOC contents (see Supplementary Text). It is possible that higher δ13Corg values associated with measurements of OM from carbonate-rich formations could be explained, at least in part, by incomplete decarbonation prior to sample analysis (Fischer et al., 2009), where δ13Ccarbonate values would drive the δ13Corg values of the measured sample to be higher. Alternatively, this difference could be driven by actual differences in lithological preservation of OM, where the presence of dolomite and ankerite minerals may play some role in structural and isotopic evolution of OM, especially at high temperature where OM has the potential to undergo exchange with reactive inorganic C pools (see sections 3 and 4; Chacko et al., 1991). Likewise, the δ13Ccarbonate can vary by a few per-mille based on depositional environment (Beukes & Klein, 1990; Des Marais, 2001), which will affect the isotopic composition of the exchangeable inorganic C (see section 4).
2.2.3. Metamorphism and pre-GOE organic carbon isotopic composition
We compared Archean δ13Ckerogen values with descriptions of metamorphic grade and measurements of H/C ratio to characterize how the carbon isotopic composition of kerogen changes with thermal and chemical evolution (fig. 4). We focused our analysis on data from de-mineralized Archean kerogens rather than whole rock TOC because those studies included the most complete descriptions of metamorphic facies. H/C ratios of TOC become less reliable as they get lower (<0.2) and are not included in datasets reported by most TOC studies. For simplification, we grouped measurements from all sub-greenschist facies together.
Immature Type II OM has a H/C ratio of ~1.2 to ~1.5 (fig. 5B), which decreases to an H/C ratio of ~1.2 to ~0.5 as it undergoes catagenesis. “Mature” OM following metagenesis into higher metamorphic grades has a H/C ratio < 0.2. Along with these stoichiometric changes as the H/C ratio decreases, we observed an increase in δ13Ckerogen values, particularly below H/C ratios of 0.2 (fig. 4A), consistent with results of a previous study (Hayes et al., 1983). Likewise, we observed a shift in δ13Ckerogen values with metamorphic grade, although shifts of more than a few per-mille in the carbon isotopic composition of Archean kerogen were not observed until greenschist facies and above (fig. 4B). Similar observations have been made before (Des Marais, 2001; Galvez et al., 2020; Hayes et al., 1983; Schidlowski, 2001) but, to our knowledge, no study has ever summarized this trend as we do in figure 4B, as a plot of δ13Ckerogen values versus qualitative description of metamorphic grade covering the full range of peak conditions.
A prior study presented an empirically derived, corrective polynomial to account for shifts in H/C ratios down to ~0.2, so that δ13Corg values could be corrected to their original values based on this proxy for thermal maturity (Des Marais, 1997). However, corrections were only presented for δ13Corg values measured from organic carbon deposited after the GOE, as there is limited kerogen data prior to the GOE — insufficient to generate a statistically meaningful polynomial fit. More importantly, interpreting this evolution as correctable by a polynomial fit conflates the effects of many reactions that could each impart a different and varying isotope effect. An improved understanding of such isotope effects is valuable for interpreting any δ13Corg values as indicators of early microbial ecosystems and processes, in particular for evaluating any useful information provided by Archean refractory carbon that has experienced high grade metamorphic conditions.
3. MODEL OF CARBON ISOTOPIC EVOLUTION THROUGH THE ROCK CYCLE
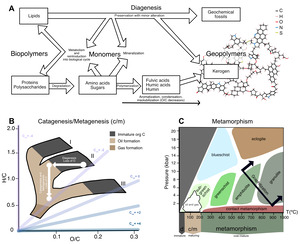
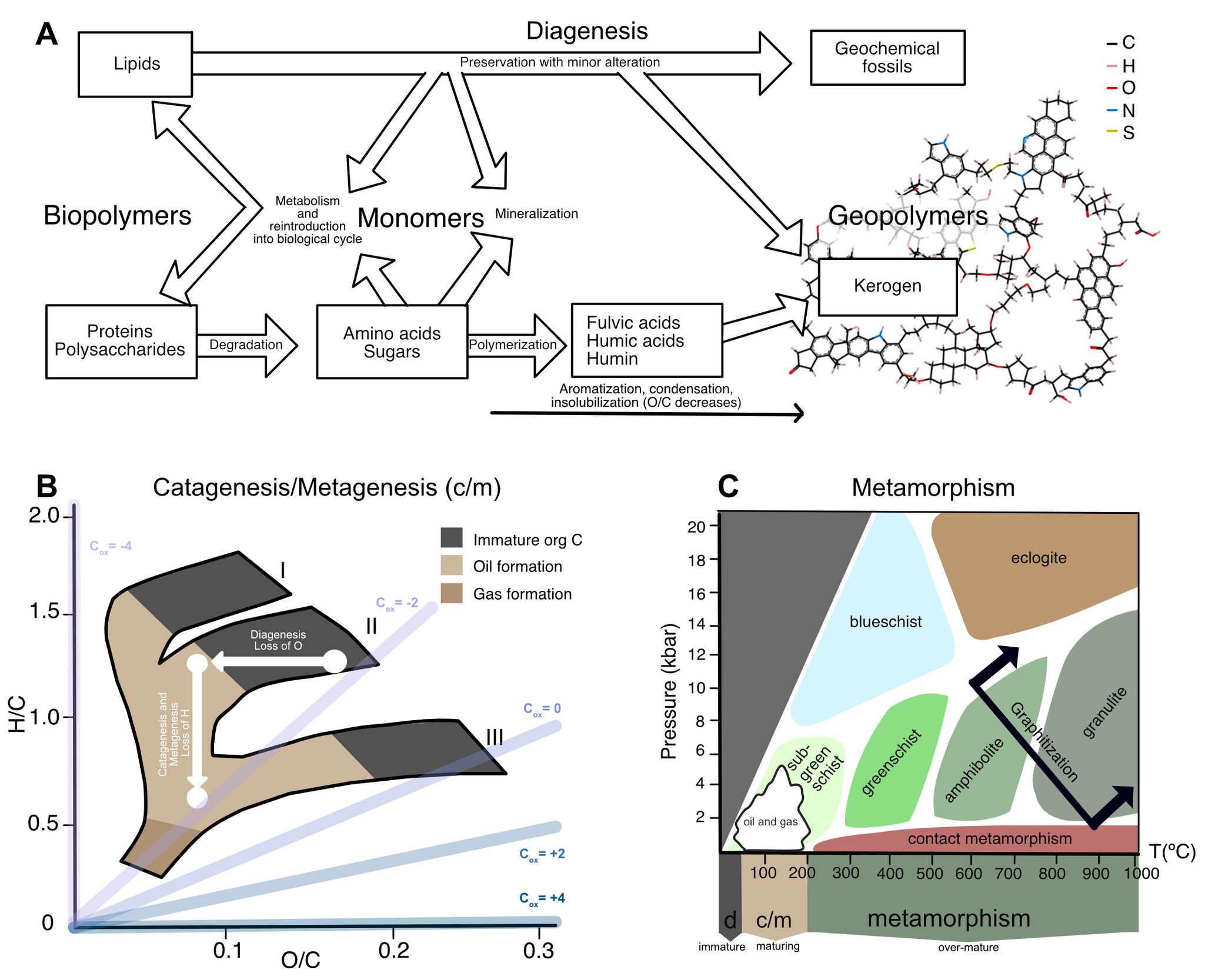
Organic carbon within Archean sedimentary rocks—even in the very best-preserved successions—has undergone a substantial amount of chemical change since initial deposition (fig. 5), from low temperature degradation (fig. 5A) to the production of oil and gas (fig. 5B) to high temperature metamorphism (fig. 5C). These changes span temperatures from 0 to >800 °C (fig. 5C), and relate to several disparate disciplines—biology, low-temperature geochemistry, petroleum geoscience, and metamorphic petrology—each with their own vocabulary to describe processes involved in the maturation of organic matter. In some cases, there exists a gap between where the focus of one field ends and the other begins. To address all these issues, we defined a combined nomenclature to describe the maturation of organic matter. We divide the evolution of organic matter into three ‘alteration regimes’: (i) Diagenesis is low-temperature alteration that occurs prior to and immediately following deposition (section 3.2; fig. 5A); (ii) catagenesis and metagenesis refer to higher-temperature processes (50–200 °C) that drive the production of oil and gas, respectively (section 3.3; fig. 5B); and (iii) metamorphism refers to processes above 200 °C that affect overmature organic matter (section 3.4; fig. 5C).
The van Krevelin diagram offers quantitative descriptions for how elemental ratios change with increasing thermal maturity. Dependent on the type of organic matter and its starting elemental and isotopic composition, the H/C ratio (>1.4 in modern, living biomass) and O/C ratio co-evolve (fig. 5B) as each kerogen type moves from low temperature diagenesis through higher temperature catagenesis and metagenesis. In the van Krevelin diagram, these two alteration regimes can be deconstructed into two vectors: the loss of O+C during diagenesis via loss of CO2 (demonstrated by an arrow moving left on the diagram), and then the loss of H+C during catagenesis via loss of CH4 and other small hydrocarbons (demonstrated by an arrow moving down on the diagram; fig. 5B). Prior studies noted that the loss of CO2, CH4 and short chain hydrocarbons should leave the OM residue enriched in 13C (Galimov, 1980; Peters et al., 1981). So-called ‘isotope effects’ are caused by differences in thermodynamic stability and rates of reaction between isotopic forms of a compound. These effects are complex, and can impart a wide range of carbon isotopic fractionations at the scale of individual atomic positions (on the order of tens of ‰ in δ13C; for example, Abelson & Hoering, 1961; Lloyd et al., 2023; O’Leary & Yapp, 1978); depending on the magnitude of fractionation and proportions of strongly fractionated atomic sites, this can lead to observable shifts in the average isotopic composition of the compound or substrate.
Kinetic isotope effects (KIEs) describe the isotopic fractionations associated with irreversible reactions and represent the preference for one isotope over another during the rate limiting step of the reaction, whereas equilibrium isotope effects (EIEs) represent the partitioning of rare isotope between two or more materials when an exchangeable system has established equilibrium. Kinetic isotope effects are often reported as a ratio of reaction rate coefficients for the unsubstituted and substituted isotopologue (kA/ka, where A is the substituted mass of the isotope and a is the unsubstituted mass; for example, 13 and 12 for the substituted and unsubstituted isotopes of C). Both equilibrium and kinetic isotope effects can be quantified by “isotopic fractionation coefficients” (α−values), which can be defined for both isotope ratios and delta values as follows:
αi−j= 13Ri13Rj=1+ δ13Ci1+ δ13Cj
where R’s and δ’s are the observed carbon isotope ratios and δ13C values, respectively, for i and j, two distinct substrates of interest. Fractionation coefficients can be converted to epsilon values (ε), which are approximately equal to the difference (∆) between the δ values of the two substrates:
ε=(αi−j−1) ≈Δ=δ13Ci−δ13Cj.
Like delta values, epsilon values are also reported in units of per-mille (‰).
3.1. Biological fixation and abiological synthesis of organic compounds
Modern sources of organic matter to marine sediments are thought to be largely derived from phytoplankton body/cell structural polymers (Tissot & Welte, 1984), with a starting composition of 50–60% proteins, 40% carbohydrates, 5–30% lipids (Burdige, 2007). Other sources of organic matter to sediment include waste products and secretions. The relative proportion of organic compounds (Huc, 1980) and the initial stoichiometry and isotopic composition of organic matter vary by organism (for example, van Dongen et al., 2002). In modern systems, extracellular polymeric substances (EPS) can be important components of microbial mats and are thought to have made up the majority of many ancient mat systems (Decho & Gutierrez, 2017; Noffke et al., 2013).
Metabolic pathways that produce biomass impart characteristic and complex effects on the isotopic composition of organic matter (and nitrogen, hydrogen, sulfur, and oxygen; Berg et al., 2010; Blaser et al., 2013). Although the dominant form of carbon fixation (Fuchs, 2011) and the respective carbon isotope fractionation in the Archean remains unknown (Flamholz et al., 2022; Wang et al., 2023), biomass fixation processes produce non-stochastic distributions of isotopes at both the site- and compound-specific level (See Supplementary Text for discussion of abiotic organic synthesis processes). For instance, glucose within cultured E.coli cells (average δ13C ~ −10‰) was found to be enriched in its 13C contents compared to lipids (average δ13C ~ −17‰; DeNiro & Epstein, 1977; Monson & Hayes, 1982), while the isotopic composition of different lipid carboxyl sites (average δ13C ~ −13‰) have δ13C values ~20‰ higher than the methylene sites (average δ13C ~ −35‰; Monson & Hayes, 1982) in those same molecules. This inter- and intra-molecular isotopic partitioning affects the isotopic composition of preserved OM, especially due to preservation biases at high levels of OM remineralization.
3.2. Deposition and diagenesis
The carbon isotopic composition of immature sedimentary OM is known to be driven by two competing factors: (i) Selective preservation of specific compounds and (ii) Structural condensation of OM, where chemical reactions that occur during maturation cause isotopic change and convert OM into a less bioavailable state. Among primary compounds, lipids are the least altered from death to preservation due to their aliphatic nature that makes them more challenging to re-mineralize. In many cases at low temperature, even original lipid biomolecules are preserved unchanged, or little changed in chemical structure (that is, they are preserved as biomarkers; fig, 5A; Killops & Killops, 2004). Only 10–40% of the structure of OM within marine sediments can be identified as primary compounds (Burdige, 2007; Spiker & Hatcher, 1984) and this number is variable with increasing sediment depth and OM maturity (Spiker & Hatcher, 1984). This suggests that there is significant remineralization of OM within particulate and dissolved organic carbon (POC and DOC). Sedimentary OM also may incorporate reworked kerogen and black carbon, or soot from burned biomass (Burdige, 2007).
Selective preservation of specific compounds can affect the δ13Corg values, due to aforementioned site- and compound-level variability. For instance, the isotopic difference between more resistant lipid molecules and more labile molecules such as carbohydrates can impart a shift in the δ13C value of the OM that is preserved within the sedimentary record (Galimov, 1980), which prior studies have demonstrated can drive the δ13Corg values of immature organic matter lower than its biological precursors by a few per-mille (Galimov, 1980; Lehmann et al., 2002; Spiker & Hatcher, 1984). Likewise, selective preservation among lipid compounds, such as straight-chain versus isoprenoid lipids, would also have an effect the carbon isotopic composition of the residual OM (Schouten et al., 1998). Thus, any variation in the relative contributions of different compounds to sedimentary organic matter will have an effect on δ13Corg values.
The condensation of organic matter during remineralization can change its isotopic composition. In modern environments, the majority of OM is known to be re-mineralized (for example, Berelson & Stott, 2003), but this may not have been true in Archean environments due to differences from the modern in particle flux rates, O2 in the water column, and organic burial efficiency (Burdige, 2007; Katsev & Crowe, 2015; Killops & Killops, 2004; Kipp et al., 2020). The oxidation of environmental organic matter is driven by glycoxidation and lipoxidation reactions such as the Maillard reaction (Moore et al., 2023), which have been mechanistically characterized in the context of food science studies (Vistoli et al., 2013), and applied to recent research on fossilization to understand how these reactions affect the preservation of molecules at the tissue-scale (Wiemann et al., 2018). Many of the reactions involved in oxidation and remineralization do not remove atoms from the system, but rather polymerize (that is, condense) the OM towards a more thermodynamically stable form. These reactions include the following: saturation of unsaturated bonds, irreversible rearrangements of Amadori products created by glycation reactions, condensation between amino acid residues and dicarbonyl groups, oxidative cross linking, cyclization, and isomerization (Killops & Killops, 2004; Vandenbroucke & Largeau, 2007; Vistoli et al., 2013). Each of these reactions impart transformations on specific sites within molecules, and so it is likely that these changes will impart isotope effects at the site level. Until this point, site-specific isotope effects for these reactions remain largely unconstrained except for several studies that found that amination and isomerization have negligible isotope effects (Baldwin & Ghatlia, 1989; Bushnev et al., 2020; Chimiak et al., 2021; Putschew et al., 1998). Oxidation reactions may be accelerated or decelerated through longer O2 exposure time within the water column (Hartnett et al., 1998), varying concentration of metals and metal catalysts (Moore et al., 2023), and interaction with and preservation by the active surfaces of minerals (Hedges & Keil, 1995; Hemingway et al., 2019; Keil et al., 1994; Keil & Mayer, 2014; Mayer, 1994).
Diagenetic reactions that promote the loss or addition of functional groups and thus change the stoichiometry of OM include decarboxylation, dehydration, deamination, and sulfurization (see Materials and Methods, Supplementary Text). Of these reactions, decarboxylation and dehydration are reactions that affect the location of immature OM on the van Krevelin diagram by changing their O/C and H/C ratios, respectively, through loss of CO2 and H2O. The loss of oxygen is clearly depicted within the van Krevelin diagram as a trend during early OM maturation and drives an overall reduction in oxidation state of C (fig. 5B). Of these reactions, the only one that would have an effect on the isotopic composition of carbon is decarboxylation.
Decarboxylation reactions operating under equilibrium conditions (which have been achieved biologically with enzymes) can have a k13/k12 as low as 0.997±0.001, but non-catalyzed carbon bond breaking events can have KIEs that range from 0.93 to 0.97 for the CO2 leaving the system (Bigeleisen & Friedman, 1949; Lewis et al., 1993; Lindsay et al., 1950; Marlier & O’Leary, 1984; O’Leary & Yapp, 1978). Although we expect that this effect could drive site-specific differences in the δ13Corg value up to tens-of-per-mille, we must also consider that this chemistry is operating on compounds that already have inherent intramolecular isotopic variation. For instance, lipids—which are known to be more resilient to early diagenesis than sugars or proteins—have a carboxyl site that is enriched in 13C compared to its other sites. Thus, in this case, there would be two opposing drivers on the δ13C of residual carbon: Decarboxylation reaction acts on carboxyl sites that begin 13C-rich compared to the isotopic composition of the other carbons (in this case, −13‰ versus −37‰ for the other sites; Monson & Hayes, 1980) but does so with a KIE that selects against 13C. Moreover, the net effect of this reaction on the average C isotopic composition of OM would be minimal (~1‰; see Materials and Methods), as carboxyl sites represent a small fraction of the total C even in the least mature OM (Ungerer et al., 2015). Thus, both selective preservation and condensation processes have not been demonstrated to drive isotopic shifts in the overall composition of OM by more than a few per-mille, which is within error of the variation found in the isotopic composition of immature organic matter in Phanerozoic sediments (Hoefs & Frey, 1976).
3.3. Catagenesis and metagenesis
Following the loss of carbon dioxide, further maturation of OM is characterized in the van Krevelin diagram as a loss of carbon and hydrogen through catagenesis (fig. 5B; Galimov, 1980; Peters et al., 1981). The loss of hydrogen is depicted within the van Krevelin diagram as a trend during later OM maturation and drives an overall oxidation of C (fig. 5B). This process was supported by a prior model of catagenetic processes that simplified the reactions driving catagenesis into a few key ones: homolytic cleavage, β-scission, H- abstraction, radical recombination, and radical isomerization (Xie et al., 2022). Xie et al. (2022) argued that the carbon isotopic fractionations associated with catagenesis could be simplified and that the main fractionating reactions driving C isotopic change were homolytic cleavage and β-scission, which both impart isotope effects of k13/k12 = 0.975±0.001 at 180 °C on the reacting carbon of a first order bond (Xie et al., 2022). As a result, the bond breaking events that produce oil and gas occur more favorably for 12C than for 13C and leave the residue enriched in 13C. The k13/k12 value can vary by 0.975±0.010 depending on the nature of the bond being broken at 180 °C, which can result in site-specific variations in the primary isotope effect that vary by up to 10‰ (Tang et al., 2000; Xie et al., 2022). Secondary and tertiary isotope effects are an order of magnitude smaller (Tang et al., 2000; Xie et al., 2022).
We applied a similar approach the one used in Xie’s study but tracked the evolving isotopic composition of the residue rather than the products of catagenesis. We model the effects of homolytic cleavage and β-scission (which we refer to together as just “homolytic cleavage” for simplicity) on a Type II kerogen model residue. Our residue begins with an isotopic composition equal to the median composition of Archean TOC (δ13Corg = −30.5‰) and an H/C ratio that starts at 1.2 and evolves to 0.1. We note that the Ungerer model describes immature Type II kerogen evolving from H/C ratios of 1.16 (Type II-A) to 0.58 (Type II-D), but H/C ratios will vary based on initial composition and kerogen type (section 3.1) and to our knowledge no studies have investigated the full range of H/C ratios that encompass alteration regimes from biomass to metamorphism. Here, we extend our model to encapsulate H/C ratios down to 0.1 to connect with the starting point of our metamorphism model (section 3.4).
We simplified the homolytic cleavage model to remove only methane at each step (1 C and 4 H, implying that hydrogen abstraction and ‘capping’ converts primary radical products to stable alkanes). In reality, the number of carbons lost with cleavage varies with phase of catagenesis, temperature, and gas formation mechanism (Milkov et al., 2020; Milkov & Etiope, 2018; Seewald et al., 1998), but higher order carbon (C2+) gases will remove more C at lower H/C ratios and with less fractionated 13C, which will achieve a similar result to the sole removal of methane applied here, but in fewer model steps (Supplemental fig. 2).
In our model, as the H/C ratio evolved from 1.2 to 0.1, 13C was distilled into the residues, driving an increase in the δ13Corg value of residual organic matter that ranged from 5 to 12‰ depending on the assumed parameters (fig. 6A&C; Supplemental Materials and Methods). Broadly, our model reproduces the results of a previously derived empirical trend between H/C ratio and the δ13Corg value that was proposed to correct δ13Corg values to their putative original values (Des Marais, 1997). Our result connects parameters related to the isotopic fractionation of carbon by catagenesis to the positive shift in kerogen carbon isotope values. This result is robust in all model iterations but accurate corrections of δ13Corg values would require more constraints on δ13Corg and the KIE of the reaction than we impose here.
3.4. Metamorphism
Finally, overmature organic matter subject to high temperatures and pressures undergoes metamorphism, which alters its structure and chemistry toward (though rarely reaching) pure crystalline graphite (fig. 5C; reviewed by Grew, 1974; Landis, 1971). At lower metamorphic grades (that is, greenschist facies and below), OM maturation towards graphitic structure is largely affected by condensation reactions such as aromatization which begin ~300 °C (Jing et al., 2007). Graphite can either grow as sheets or as stacks and remain largely unchanged in structure once the source rock reaches temperatures ~400 °C (Wopenka & Pasteris, 1993).
However, as temperatures and pressures increase and rocks undergo amphibolite and granulite facies metamorphism, carbon from OM can exist in a fluid and mobile phase (Kipling et al., 1964). These carbons can exchange with carbonate-derived C in the surrounding fluid, before recrystallizing through a process called Ostwald ripening where smaller particles preferentially dissolve and larger particles grow, mediated by carbon transfer through pore fluids (Dunn & Valley, 1992; Kelly, 1981). Previous studies of amphibolite and granulite facies marbles have shown that this process is accompanied by carbon isotope equilibration between organic matter and carbonate phases in the host rock (αcarbonate-OM = 1.006 at 600 °C; Chacko et al., 1991; Dunn & Valley, 1992; Kitchen & Valley, 1995; Valley & O’Neil, 1981). However, the kinetics of carbon exchange with graphite are slow and equilibrium is unlikely to be obtained until peak metamorphic temperatures of 500–600 °C (Valley & O’Neil, 1981). As such, after formation, the bulk of the graphite particle is unlikely to exchange again at these temperatures and pressures (Evans et al., 1969; Scheele & Hoefs, 1992).
Some of these graphite crystals have been observed within the oldest and most metamorphosed Archean rocks, such as those from Akilia (McKeegan et al., 2007; Mojzsis et al., 1996), Isua (Ohtomo et al., 2014; Rosing, 1999), and possibly Nuvvuagittuq (Papineau et al., 2011). However, for most of the Archean samples included in this compilation, which generally reached peak metamorphic temperatures lower than this threshold, it is unlikely that any of the OM reached full equilibration, but rather equilibrated partially with co-existing carbonate minerals and the DIC in the pore fluid. The expectation is that this will lead to substantially higher carbon isotope values with increasing metamorphic grade (Des Marais, 2001; Dunn & Valley, 1992; Galvez et al., 2020; Schidlowski, 2001).
To model this final stage of maturation, we modeled the exchange of a pool of OM with a starting δ13Corg value equal to the average final δ13Corg value for OM from the catagenesis model (δ13Corg = −26‰) and a carbonate pool with initial δ13Ccarbonate values that range from −6 to 0‰ (fig. 6B; see Supplementary Materials and Methods), which can vary depending on water depth (Beukes & Klein, 1990; Des Marais, 2001). Likewise, the maximum achievable δ13Corg value of equilibrated OM is limited by the EIE between DIC and OM, which we estimated to have a δ13Corg = −6‰ for a δ13Ccarbonate = 0. The maximum δ13Corg calculated by this model is achieved by solving for a system that exists at full equilibration with an infinite reservoir of inorganic carbon; that is, for the limiting case where DIC within seawater and pore fluids is far larger than the amount of sedimentary organic matter in the rock record that is exchanging with it.
For a case where δ13Ccarbonate value = −6‰, at 20% exchange (80% of the original OM remains un-exchanged), the average δ13C value of the OM was −22.7‰ (fig. 6B; Supplemental Materials and Methods). Likewise, at 40, 60 and 80% exchange, the δ13C values of the OM were −18.6‰, −14.4‰ and −10.2‰, respectively (fig. 6B). The results for models produced with other δ13Ccarbonate values are included in figure 6B. This result is consistent with the δ13C values of organic matter in high grade metamorphic samples from the Archean and contextualizes these values within a mechanistic explanation of the shifting carbon isotopic composition associated with graphite grain growth during high grade metamorphism.
3.5. Comparison of model to empirical data
Empirical constraints from prior studies and constraints imposed by the model described above can be used to relate changes in the δ13Corg values of organic matter to the chemical changes that occur through processes of thermal maturation (figs. 4, 5, & 6). Notably, our model connects distinct regimes of geochemistry, stitching together changes that have been previously characterized separately by organic geochemists, petroleum geologists, and metamorphic petrologists (fig. 4C). The changes in δ13Corg that we described in our model are also consistent with a prior study that measured Phanerozoic δ13Corg values across a metamorphic gradient in a transect within the Swiss Alps (fig. 6C; Hoefs & Frey, 1976), especially when the offset between Archean and Phanerozoic δ13Corg values are considered.
4. REINTERPRETING ARCHEAN RECORDS
Our mechanistic model of the evolution of δ13Corg values with changes in the O, H, and C ratios of OM through the rock cycle provides a framework to revisit the carbon isotopic composition of Archean organic matter. Major shifts in δ13Corg values during end-stages of metagenesis and, especially, metamorphism through C exchange between OM and DIC can provide an explanation for 13C-enriched carbon isotope values of amphibolite facies OM within Isua and Akilia samples (section 4.1). While we cannot rule out the possibility that the bimodality in the δ13Corg values from the late Archean may be the result of variable amounts of alteration, in section 4.2 we explore the alternative hypothesis that this bimodality may be the caused by Archean metabolisms that impart distinct isotopic fractionations on biomass.
4.1. Isua, Akilia and Hadean zircons
The carbon isotopic composition of OM found within Isua and Akilia samples spans a range of values from a minimum of ~−30‰ to a maximum of −5‰ (Grassineau et al., 2006; Mojzsis et al., 1996; Ohtomo et al., 2014; Rosing, 1999; Shimoyama & Matsubaya, 1992; Strauss & Moore, 1992; Tashiro et al., 2017; Ueno et al., 2002; van Zuilen et al., 2003). Our model provides a mechanistic explanation for how biogenic organic matter could have reached the higher end of this distribution of carbon isotope values by exchanging with solid carbonates or fluid DIC. Indeed, to achieve the highest δ13Corg values (~ −5‰) under amphibolite facies metamorphic conditions from a 13C-deplete source, the OM would have to undergo full equilibration with reactive carbon in the surrounding fluid (fig. 6B&C). Likewise, moderately high values (~−15‰) within these samples may be the result of biogenic OM that has undergone extreme catagenesis and metagenesis but only limited or no equilibration with reactive inorganic C. However, the idea that high δ13Corg values may be measurements of completely abiotic carbon (i.e., with no pre-metamorphic biological substrates) cannot be ruled out. Higher δ13Corg values overlap with the carbon isotopic composition that is expected of abiotic graphite that formed from mantle-derived carbon within metamorphic fluids (Taylor, 1986; Weis et al., 1981), as well as some meteoritic organics (Glavin et al., 2018; see Supplementary Text).
Low δ13Corg values within samples that have undergone amphibolite facies metamorphism could be the result of several potential scenarios. First, the OM characterized from these samples could be representative of original Archean biogenic OM that was prevented from equilibrating with the reactive carbon pool, either due to armoring by mantling minerals or fluid-absent metamorphism. Second, these values may represent rare grains that failed to dissolve, exchange, and re-precipitate as crystalline graphite because metamorphic carbon isotope exchange involving graphitic materials appears to require dissolution and re-precipitation during Oswald ripening, not simple solid-state carbon mobility. Finally, low δ13Corg values may derive from contamination from more recent biogenic OM. Regardless of the explanation, we suggest that future studies focus on the textural and isotopic differences between re-crystallized graphite and more amorphous, overmature kerogen: Studies of kerogen that have not yet undergone crystallization and exchange, especially alongside measurements of the H/C ratios, can ensure that studies are targeting samples that have undergone the least amount of alteration and anchor that choice within the context of the van Krevelin diagram.
4.2. Low δ13Corg values and explaining the bimodality
The lowest carbon isotope values for Archean organic carbon occur within relatively un-metamorphosed units dating to ~2.7 Gya (Fischer et al., 2009; Kaufman et al., 2007; Slotznick & Fischer, 2016; Strauss & Moore, 1992; Thomazo et al., 2009; Williford et al., 2016; Yoshiya et al., 2012), such as the Tumbiana formation within the Fortescue group, which have measured minimum δ13Corg values as low as −60.9‰ (Slotznick & Fischer, 2016). These measurements contribute to the bimodal trend observed by this study for δ13Ckerogen, which are not unique to the Tumbiana Formation but are also present in formations across the Fortescue and Hamersley groups, and preserved even when the dataset was subsampled for sampling bias and geographic variation (fig. 3). Kernel density estimates of δ13Corg value distributions revealed higher values overall for data from the co-occurring Kaapvaal, Dhawar or Belingwe craton data, but with some multimodalities (fig. 3).
The presence of multimodal features in the Archean carbon isotope dataset implies the existence of multiple dominant environmental mechanisms to create them. This is the case for modern organic compounds, where compounds that are formed by two or more metabolic pathways demonstrate intra-site variations in their isotopic contents that can be traced back to metabolic process (for example, Lloyd et al., 2023). Likewise, a similar analogy can be made to a well-understood and widely studied difference in δ13Corg values between geologically recent C3 and C4 plants (reviewed in Hayes, 2001), which utilize distinct photosynthetic processes. It is only appropriate then that multimodalities found within the δ13Corg values of Archean OM are interpreted as records of two-or-more distinct processes.
We cannot rule out that variations in δ13Corg values could be driven by post-depositional environmental factors (see section 2), as some of the data points within these units have H/C ratios as a low as 0.14 and 0.25 (corresponding to δ13Corg values of −41‰ and −51.2‰, respectively). Multimodalities in δ13Corg values could be the result of OM that has been altered more and less. However, it is unlikely that diagenetic or catagenetic processes drove these carbon isotope values to be lower than their starting compositions by a few per-mille (see section 3; fig. 6A&C). Thus, it is necessary to consider that these multimodalities may reflect variations in biological carbon fixation processes and their environmental abundance throughout Earth’s history.
Variations in δ13Corg values of Archean OM may reflect two or more populations of biomass formed by distinct metabolisms, each dominant in different Archean environments, which each impart their own diagnostic isotopic fingerprint. While there are currently seven known pathways capable of carbon fixation in the biosphere, there is no consensus for when these pathways emerged. Based on arguments of uniformitarianism, past studies have suggested that low δ13Corg values may be linked to aerobic methanotrophy (Hayes, 1983), or the anaerobic oxidation of methane (AOM) or the reductive acetyl Coenzyme A (CoA) pathway (Wood-Ljungdahl pathway; Slotznick & Fischer, 2016).
Finally, it is possible that these low δ13Corg values could be due to the abiotic formation of isotopically deplete organic matter. A prior study proposed that low δ13Corg values in the late Archean could be caused by a photochemical mechanism, where the low carbon signature is dominated by an abiotically-produced organic rich haze that produces particulates that get incorporated into the sedimentary record (Pavlov et al., 2001).
5. CONCLUSIONS
The Archean carbon isotope record reflects a biosphere and a geosphere that were distinctly different from those of the modern (Estes et al., 2019; Fuchs, 2011; McCollom, 2013; Slotznick & Fischer, 2016; Sutherland, 2016), and can have implications for biogenic vs. abiotic carbon cycling, and first order aspects of metabolism. However, Archean organic matter is subject to profound changes in chemical and isotopic composition — in extreme cases equaling the amplitude of metabolic carbon isotope fractionations — as it evolves through the rock cycle, such that all such measurements must consider in some detail the combined influences of diagenesis, catagenesis and metagenesis, and metamorphism.
We suggest that it should become routine for such studies to pair measurements of δ13Corg values with measurements of OM stoichiometry (H/C and O/C ratios) so that samples can be placed in the context of the van Krevelin diagram. Similarly, carbon isotope measurements could be paired with studies of OM structure (that is, amorphicity) to constrain the processes that have affected it at higher metamorphic grades. More generally, future studies should wrestle with the potential chemistries that can create, exacerbate, and minimize multimodalities within carbon isotope datasets, taking into consideration the chemical isotope effects that occur between oxidized and reduced, as well as condensed or gaseous C species. Such effects are large and potentially impactful for the isotopic composition of OM residues. Future studies should also explore the effect of these processes on intra-site carbon isotope variations within maturing organic matter (for example, Lloyd et al., 2021), which will be imperative to answering both long-standing historical as well as more modern scientific questions. Finally, new techniques available to model the evolution of microbial metabolisms (for example, Goldford et al., 2017, 2019; Goldford & Segrè, 2018; Raymond & Segrè, 2006) can help to constrain the origins of first order features of the Archean carbon isotope record, such as the pronounced bimodality in δ13Ckerogen documented here, and in this way help to ordinate the evolution of metabolic mechanisms during the Archean.
The study of refractory carbon is not only relevant to improving our understanding of the deep past on Earth, but also to recent, ongoing, and planned extraterrestrial sample return missions like Perseverance (to Mars), OSIRIS-REx (to asteroid Bennu) and Hayabusa2 (to asteroid Ryugu). These missions aim to characterize material for its abiotic organic chemistry, and/or for signs of past or present life. It is possible some of these samples will not contain diagnostic molecular information that can ally them with specific organisms or processes. Based on the meteorite record, we should expect that most of the OM that will be found within extraterrestrial samples will be macromolecular and insoluble. It is important to understand how to interpret carbon isotope values of this OM, and how they may be modified with chemical and thermal maturation, in the hopes that we will correctly recognize the remains of extraterrestrial life should we be fortunate enough to sample it.
Acknowledgments
We thank the following individuals for conversations that have contributed to various stages of this project: Tanja Bosak, Guannan Dong, Katherine Freeman, Katherine French, Nami Kitchen, Usha Lingappa, Max Lloyd, Gordon Love, Juliet Ryan-Davis, NiveditaThiagarajan, Jasmina Wiemann, and Hao Xie. We are grateful to Alex Sessions, Jordon Hemingway, Stephen Mojzsis, and the anonymous Reviewer for their suggestions in the revision process. Thanks to Karen Craddock, Diane Giroux, Ethel Liske, and Trevor Teed for conversations regarding the history and naming of the “Slave Craton” and how Earth scientists can work to improve how we approach the intersection of our work with indigenous tradition, and other communities more broadly. S.S.Z. was supported by the NSF Graduate Research Fellowship, the Simons Foundation, and the Resnick Sustainability Institute. This work was conducted on the ancestral lands of the Gabrielino-Tongva and Chumash people.
Author contributions
S.S.Z. conceived of the project, performed the literature review, compiled and analyzed the data, designed the mechanistic model, and wrote the paper. W.W.F. participated in investigation, data analysis, interpretation, and paper revisions. N.L. aided in designing the mechanistic model and collating the data compilation. K.R.M. compiled data related to taphonomy and sedimentology and wrote the related sections. J.E.G. contributed interpretation with regards to biological implications of this study. J.M.E. participated in investigation, data analysis, interpretation, mechanistic model design, and paper revisions.
Data availability
The full data compilation for this study, as well as the spreadsheet associated with the maturation model can be found in a GitHub repository (Zeichner, 2023). Additional materials and methods for the model, and supplemental table and figures are included in a supplementary document.
Supplementary Document
https://doi.org/10.5281/zenodo.8280728
Editor: C. Page Chamberlain, Associate Editor: Ann Pearson