1. Introduction
The term ‘A-type’ granite was introduced by Loiselle and Wones (1979) to describe granitic rocks that are found at rift zones and within stable continental blocks. Two compositionally distinct A-type granites were initially characterized. One sub-type is described as being derived by fractional crystallization of alkali basalt with minimal crustal interaction whereas the other sub-type is derived by interaction of crustal melts and alkali basalt. Eby (1990, 1992) classified the basalt-derived sub-type as A1 and the crust-basalt-derived subtype as A2. Since the introduction of the A-type granite concept there has been significant interest in understanding the petrogenesis of each sub-type and constraining the petrological parameters under which these granites can develop as they are commonly associated with rare metal deposits and anorogenic tectonic settings including flood basalt provinces and orogenic collapse (Bonin, 2007; Černý, et al., 2005; Chakhmouradian & Zaitsev, 2012; Clemens et al., 1986; Collins et al., 1982; Dall’Agnol et al., 2012; Dostal, 2017; Eby, 1990; C. D. Frost & Frost, 1997; Landenberger & Collins, 1996; Patiño Douce, 1997). Although there is debate on the merit or usefulness of the ‘A-type’ concept, it has shown resilience and is still widely understood in spite of the ambiguity in its petrogenetic meaning (Bonin, 2007; C. D. Frost & Frost, 2011; Hogan et al., 1992).
The Seychelles microcontinent (~44,000 km2) is located at the northern end of the Mascarene plateau in the western Indian Ocean. It is a submerged fragment of continental crust that rifted from India during the Early Paleogene (Ashwal, 2019; Collier et al., 2008; Hammond et al., 2013; Torsvik et al., 2013). The outer islands of the Seychelles are mostly atolls with carbonate rocks and sandy shoals, but the inner islands are composed principally of A-type granitoids with subordinate mafic intrusive and silicic volcanic rocks (Ashwal et al., 2002; Dickin et al., 1986; Shellnutt et al., 2020; Weis & Deutsch, 1984). Nearly all of the granitic inner islands are Neoproterozoic (750−810 Ma) in age however the islands of Silhouette and North Island (Ile du Nord) are syenitic and Early Paleogene (60−65 Ma) in age (Dickin et al., 1986; Ganerød et al., 2011; Shellnutt et al., 2017, 2020; Tucker et al., 2001). The Silhouette and North Island syenite complexes are considered to be petrogenetically associated with the eruption of the Deccan Traps and the passage of Indian plate over the Réunion hotspot (Devey & Stephens, 1992; Dickin et al., 1986; Gaina et al., 2015; Hammond et al., 2012; Owen-Smith et al., 2013). The island of Silhouette is composed of a volcanic unit (trachytic tuff) and a composite syenite and riebeckite granite ring complex (Baker, 1963; Dickin et al., 1986; Stephens, 1996). In contrast, North Island appears to be a single plutonic unit and therefore offers an opportunity to examine the formation of a ‘self-contained’ A-type granitoid associated with a flood basalt province.
North Island is low-lying and the smaller of the two Early Paleogene islands. It is composed of diorite, syenite, microsyenite, and microgranite and cross-cut by mafic dikes (Baker, 1963). The rocks are well exposed and readily accessible for collection. Although syenite predominates, in the southeast of the island (Congoment) there is a distinct region of intermixed diorite and syenite (Baker, 1963; Owen-Smith et al., 2013). Previous studies conclude that the North Island Complex (NIC) was derived by fractional crystallization of a mafic parental magma from the Réunion hotspot at low pressure (0.1 GPa). It is thought that the NIC and alkaline silicic rocks of Silhouette represent two eruptive centres of a single magmatic system (Dickin et al., 1986; Owen-Smith et al., 2013). However, zircon geochronology suggests that the rocks of North Island are slightly (~0.2 Ma to ~2.0 Ma) younger than the rocks of Silhouette and that they may be petrogenetically distinct magma systems (Ganerød et al., 2011; Shellnutt et al., 2017). Furthermore, the metaluminous nature of the syenitic rocks contrasts with the notion that they evolved at relatively low pressure as this tends to generate peralkaline compositions (C. D. Frost & Frost, 2011; Macdonald et al., 2011; Macdonald, 2012; Shellnutt et al., 2011, 2016; White et al., 2009). It is possible that contamination by crustal material (melt, fluids) may be responsible for the metaluminous nature of the syenites, but evidence for contamination is limited to non-existent (Owen-Smith et al., 2013). Therefore, an examination of the NIC can provide additional information on the nature of the youngest igneous rocks of the Seychelles microcontinent and on the petrogenesis of A1-type within-plate granitoids in general.
In this study, we present spot age zircon U-Pb geochronology and Hf isotopes, mineral chemistry, whole rock geochemistry, and whole rock Sr-Nd isotopes of diorites, syenites, and microsyenite from the North Island complex of the Seychelles. The data are used to constrain the tectonomagmatic evolution of the NIC within the context of Early Paleogene rifting of the Seychelles microcontinent from western India and the emplacement of the Deccan Traps. Moreover, we evaluate the importance of crystallization pressure in the formation of metaluminous A1-type granitoids.
2. Geological Background
The Inner Islands of the Seychelles consist of three groups that are distinguished based on their geography, composition, and age (fig. 1). The majority of the Inner Islands are Precambrian in age and fall within two groups: the Mahé group (Mahé, St. Anne, Moyenne, Longue, Cerf, Anonyme, Sud-est, Mamelles, Concepcion, and Thérèse) and the Praslin group (Praslin, Cousine, Cousin, Aride, Curieuse, Les Soeurs, La Dique, Mariane, Recifs, and Fregate). The islands of the Mahé group are, granitic, grey in color, and have zircon U-Pb ages that range from 750–765 Ma. In comparison, the Praslin island group has a similar but more restricted age range (750–760 Ma) and they are composed of granites that are red to pink in color (Ashwal et al., 2002; Shellnutt et al., 2020; Tucker et al., 2001). An outlier of the Praslin group is Recifs as it yielded an older age of 809 ± 1.9 Ma (Tucker et al., 2001). The origin of the Mahé and Praslin groups is debated as they are considered to be either related to an Andean-type active continental margin of Rodinia (Ashwal et al., 2002; Tucker et al., 2001) or an anorogenic/post-collisional setting (Shellnutt et al., 2020; Weis & Deutsch, 1984).
The third group of islands is composed of Silhouette and North Island (fig. 1B). North Island and Silhouette are the youngest islands and located at the western edge of the Inner Islands (Baker, 1963; Owen-Smith et al., 2013). The islands are composed primarily of greenish or buff colored syenitic rocks that yielded K-Ar, Ar-Ar, and U-Pb ages of 60−65 Ma (Baker & Miller, 1963; Dickin et al., 1986; Ganerød et al., 2011; Shellnutt et al., 2015, 2017; Stephens, 1996). There is a subordinate amount of mafic rocks on both islands (Devey & Stephens, 1992; Owen-Smith et al., 2013). Silhouette (20.1 km2) is located ~20 km NW of Mahé and is considered to be a ring complex of syenite and granite with two outcroppings of volcanic rocks along the easternmost portion of the island at Pointe Varreur-Pointe Ramsse and Pointe Zeng Zeng (Baker, 1963).
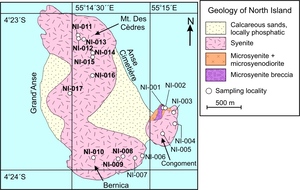
North Island is located five kilometres north of Silhouette and covers an area of 2.01 km2 (fig. 2). The north and south regions of the island are rocky steep slopes whereas the eastern and western regions (Grand’Anse and Anse Bonnet Carret) are broad, calcareous, and sandy plateaux. The principal rock-type of North Island is buff grey syenite, but there is a small exposure of darker fayalite-biotite-bearing gabbro/diorite on the eastern side of the Congoment promontory (fig. 3A, B, C). Furthermore, there are also dark-colored veins, microgranular enclaves, and dikes of porphyritic microsyenite observed around Mt. des Cèdres and along the cross-island trail (fig. 3D). Seventeen samples were collected across North Island and include diorite, syenite, and a microsyenite vein (fig. 2).
3. Petrography
3.1. Diorite
The diorites (NI-001, NI-002) are coarse grained and granular and composed primarily of feldspar (~60 vol.%), clinopyroxene (~10 vol.%), fayalite (~10 vol.%), biotite (10–15 vol.%), apatite (< 5 vol.%), and Fe-Ti oxide minerals (< 5 vol.%). There are accessory (≤ 1 vol%) amounts of zircon. Some feldspars have polysynthetic twinning whereas others have Carlsbad twinning. The twinning differences indicate that both plagioclase (oligoclase) and alkali feldspar (sanidine) are present in the diorites. Texturally, the plagioclase and alkali feldspar are euhedral to subhedral in shape and both feldspars are up to 1.5 cm long. The mafic minerals are primarily found within clusters interstitial to the feldspars where coarse grained euhedral biotite surrounds subrounded clinopyroxene and fayalite (fig. 4A, B). Euhedral acicular and hexagonal apatite and amorphous Fe-Ti oxide minerals (ilmenite and magnetite) are also within the mafic mineral clusters.
3.2. Syenite
The syenites are composed primarily of alkali feldspar (60−70 vol.%), clinopyroxene (5−15 vol.%), fayalite (≤ 10 vol.%), biotite (≤ 10 vol.%), amphibole (≤ 5 vol.%), apatite (< 5 vol.%), and Fe-Ti oxide minerals (< 5 vol.%). There are accessory (≤ 1 vol%) amounts of quartz, orthopyroxene, plagioclase, titanite, and zircon. The rocks are fresh although some show evidence of low temperature deuteric alteration of feldspar, fayalite, and biotite. The modal abundance and textures have significant spatial variability. The syenites from southeastern North Island (NI-001−NI-008) are coarse grained and granular whereas the majority of rocks from the west and north (NI-009–NI-017) are medium grained and granular (fig. 4C–F). Moreover, the amount of fayalite decreases from east to west and the amount of amphibole increases from east to west. Generally, the alkali feldspars (albite, anorthoclase, sanidine) have irregular elongated shapes that are 0.1–1.5 cm long with a consertal texture and the majority has microperthite or perthite exsolution lamellae whereas a minority has Carlsbad twinning. In the coarse grained rocks, the mafic silicate and oxide minerals commonly form clusters that are ≤ 1 cm wide and interstitial to the alkali feldspars. The mineral clusters are composed of clinopyroxene, fayalite, euhedral biotite with zircon inclusions, Fe-Ti oxide minerals (ilmenite and magnetite), and euhedral apatite, with euhedral to subhedral amphibole more commonly present in the western samples (fig. 4C, D). The medium grained rocks do not have large mineral clusters and instead tend to have single mafic minerals interstitial to the alkali feldspar (fig. 4E, F). The clinopyroxene is typically subhedral (rounded) with individual crystals up to ~5 mm long. The fayalite crystals are sub-round to equant in shape and up to ~3 mm wide. Fayalite is identified by its high relief and the presence of iddingsite around the crystal boundaries as well as in mineral fractures. Within the mineral cluster it is common for biotite, Fe-Ti oxide minerals, and apatite to be associated whereas clinopyroxene or fayalite are often but not always within the cluster. Quartz and plagioclase, identified by polysynthetic twinning, are rare, but, if present, are small (< 0.5 mm wide) and interstitial to the alkali feldspar. Titanite and zircon are not abundant and identified based on their relief, color, and birefringence.
3.3. Microsyenite
The microsyenite (NI-004V) is medium to fine grained and granular. It is composed of alkali feldspar (70–75 vol.%), quartz (~10 vol.%), amphibole (5–10 vol.%), biotite (≤ 5 vol.%), clinopyroxene (< 5 vol.%), and Fe-Ti oxide minerals (≤ 5 vol.%). There are accessory (< 1 vol.%) amounts of zircon and apatite. The rock is fresh although many of the feldspar crystals show minor saussurite alteration. The alkali feldspars are euhedral to subhedral and with the majority (60–70%) having perthite exsolution texture and a minority (30–40%) showing cross-hatched twinning. All other minerals are interstitial to the alkali feldspar. The mafic minerals do not cluster and are randomly distributed. The amphibole and clinopyroxene are distinguished by their cleavage, but have similar size and textures (subhedral to euhedral). The biotite is euhedral and contains zircon inclusions. Fe-Ti oxide minerals are spatially associated with the mafic minerals and typically have euhedral to subhedral shapes.
4. Analytical Methods
4.1. Electron Probe Micro-analyzer
The mineralogical investigation was carried out by a field emission electron microprobe (JEOL EPMA JXA-8500F) equipped with five wave-length dispersive spectrometers (WDS). Each rock specimen was sliced to a 1-inch diameter size and mounted into epoxy resin, and then polished. Secondary- and back-scattered electron images were used to guide the analysis on targeted positions of minerals. A 2 µm defocused beam was operated for quantitative analysis at an acceleration voltage of 12 kV with a beam current of 6 nA. The measured X-ray intensities were corrected by ZAF-oxide method using the standard calibration of synthetic and natural chemical-known standards with various diffracting crystals. They include: wollastonite for Si-Kα, with TAP crystal, rutile for Ti-Kα with PET crystal, corundum for Al-Kα (TAP), chromium oxide for Cr-Kα (PET), hematite for Fe-Kα with LiF crystal, manganese oxide for Mn-Kα (PET), periclase for Mg-Kα (TAP), nickel oxide for Ni-Kα (LiF), zinc oxide for Zn-Kα (LiF), wollastonite for Ca-Kα (PET), albite for Na-Kα (TAP), sanidine for K-Kα (PET), fluorite for F-Kα (TAP), and apatite for P-Kα (PET). Peak counting times for F was 20s, and others were 10s, respectively. The measured values of the reference standards were within 1% of accepted values. The mineral chemistry results are presented in supplementary table S1(https://doi.org/10.17632/8mkngdftfp.2).
4.2. LA-ICP-MS/MS Geochronology
The analytical method is summarized in table S2, following the reporting template of Horstwood et al. (2016), and detailed in Supplementary Materials, Methods. Zircon U-Pb dating was carried out using an Agilent 8900 Triple Quadrupole ICP-MS (ICP-MS/MS; Agilent, Santa Clara, CA, US) coupled to a Teledyne CETAC Analyte Excite Plus laser-ablation system (Teledyne CETAC, Omaha, NE, US), that utilizes a 193 nm ArF excimer laser, at the Department of Earth Sciences, National Taiwan Normal University. The U-Th-Pb isotope analysis was conducted in MS/MS mode; the isobaric interference of 204Hg on 204Pb was minimized using a gas-phase charge transfer reaction with NH3 reaction gas in the reaction cell (Woods, 2017). The spot size of the laser used in this study is 25 μm in diameter. 91500 zircon and NIST SRM 610 glass reference materials were used for an external calibration of U/Pb and Pb/Pb isotope ratios, respectively. Data reduction was conducted off-line, using an in-house excel spreadsheet for U-Th-Pb age data reduction (Hattori et al., 2017; Sakata et al., 2014). Using the Hg-corrected 204Pb, a correction for common Pb was made on the basis of the 204Pb method (Stern, 1997) and the model for the common Pb compositions (Stacey & Kramers, 1975).
During the analyses, Plešovice (Sláma et al., 2008) and AS3 (Paces & Miller, 1993) zircons were used as secondary standards for data quality assessment (Supplementary Materials, Data). The 206Pb/238U age weighted-mean age for Plešovice zircon determined in this study yielded 333.2 ± 4.7 Ma (n = 4; MSWD = 1.3) and is consistent with the age of 337.13 ± 0.37 Ma reported by Sláma et al. (2008). The 207Pb/206Pb weighted-mean age for AS3 zircon determined in this study yielded 1094 ± 39 Ma (n = 4; MSWD = 0.59) and is consistent with the age of 1099.1 ± 0.5 Ma reported by Paces and Miller (1993). The data was plotted using Isoplot 3.75 (Ludwig, 2012) and the results can be found in table S3.
4.3. Zircon Hf Isotopes
Zircon Hf isotope analyses were carried out on the zircon crystals analyzed for U-Pb dating reported by Shellnutt et al. (2017). A Photon Machines Analyte G2 laser-ablation microprobe attached to a Nu Plasma HR multi-collector ICP-MS was used at the Institute of Earth Sciences, Academia Sinica in Taipei. The Nu Plasma HR MC-ICP-MS features a unique geometry with a fixed detector array of 12 Faraday cups and 3 ion counters. For this study, masses 172, 175, 176, 177, 178, 179 and 180 were simultaneously analyzed. Data were considered for isobaric interferences and normalized to 179Hf/177Hf = 0.7325, using an exponential correction for mass bias. Isobaric interferences of 176Lu and 176Yb on 176Hf were corrected by measuring the intensities of the interference-free 175Lu and 172Yb isotopes and using appropriate 176Lu/175Lu and 176Yb/172Yb ratios to calculate 176Lu and 176Yb values. In the method of Griffin et al. (2000) it is assumed that ƒHf = ƒYb = ƒLu (ƒ is the mass fractionation coefficient) and the mass bias obtained for Hf is also applied to the Yb and Lu mass bias correction. Griffin et al. (2000) tested this correction method by analyzing solutions of JMC475 spiked with Yb and JMC475 spiked with Lu. The ‘true’ values for 172Yb/176Yb and 175Lu/176Lu were adjusted to give the ‘true’ 176Hf/177Hf of JMC475. The Yb and Lu isotopic compositions derived from the solution analyses were then used to correct the laser analyses. The instrumental conditions and data acquisition are similar to Griffin et al. (2000). The accuracy and precision of the method are shown by the analyses of reference zircons (Mud Tank, Plešovice, 91500, and TEMORA) and the accuracy of the correction procedure was demonstrated for 176Yb/177Hf ≤ 0.1 and 176Lu/177Hf ≤ 0.10, encompassing the vast majority of typical zircon (176Yb/177Hf ≤ 0.1 and 176Lu/177Hf ≤ 0.002; Belousova et al., 2002; Griffin et al., 2004, 2006) and the zircon samples in this study (176Yb/177Hf ≤ 0.1 and 176Lu/177Hf ≤ 0.0030).
The Photon Machines Analyte G2 excimer laser ablation system delivers a beam of 193 nm UV light with a wide range of fluences (<1 to 40 J/cm2). Most analyses were carried out with a beam diameter of 50 μm, 8 Hz repetition rate, and energy of ~8–9 J/cm2. This resulted in total Hf signals of up to ~10 V, depending on the precise conditions and Hf contents. Analyzing times were ~2 minutes including 30 seconds of the background noise and ~80 seconds of the sample ablation. The He carrier gas of ~0.9 L/min (MFC1 = ~0.7 L/min and MFC2 = ~0.2 L/min) transported the ablated sample from the laser-ablation cell via a mixing chamber where it was mixed with Ar of ~0.7 L/min prior to entering the ICP-MS torch. The He gas could substantially reduce the deposition of ablated material onto the sample surface and greatly improve transport efficiency, and thus increase the signal intensities. Laser-ablation analyses were carried out using time-resolved analysis (TRA) software, in which the signal for each mass and ratio is displayed as a function of time during the analysis. This allows the more stable portions of the ablation to be selected for analysis, before the data are processed to yield the final results. Typical within-run precision (1σ) on the analysis of 176Hf/177Hf is better than ±0.000030, equivalent to an uncertainty of ~1 epsilon unit. International zircon standard Mud Tank was used as the primary external reference material to monitor the condition of facilities and Plešovice, 91500, and TEMORA served as the secondary external reference materials for data quality control. They have long-term average 176Hf/177Hf values of 0.282496 ± 29 (2σ, n = 636), 0.282483 ± 21 (2σ, n = 84), 0.282314 ± 23 (2σ, n = 40), and 0.282689 ± 30 (2σ, n = 42), respectively, all in accordance with those of 0.282504 ± 44, 0.282482 ± 13, 0.282307 ± 31, 0.282680 ± 31 reported in Woodhead and Hergt (2005), Wu et al. (2006), and Sláma et al. (2008). The εHf(t) values (parts in 104 deviation of initial 176Hf/177Hf isotopic ratios between the sample and the chondritic uniform reservoir), the depleted mantle model ages (TDM), with the assumption that the protolith of the zircon’s host magma had the average continental crustal 176Lu/177Hf ratio of 0.015, were calculated after Griffin et al. (2004) using the 176Lu–177Hf decay constant of 1.867 × 10−11 (Söderlund et al., 2004), and the chondritic values of 176Hf/177Hf (0.282772) and 176Lu/177Hf (0.0332) from Blichert-Toft and Albarède (1997). The results are presented in table S4.
4.4. X-ray Fluorescence Spectrometry
Rock samples were cut into small pieces using a diamond-bonded steel saw and were then crushed in a steel jaw crusher. The crusher was rinsed and cleaned with de-ionized water after each sample was processed. The crushed samples were pulverized to 200 mesh in an agate mill. After drying the samples at 105 ℃ they were heated to 900 ℃ to determine loss on ignition (LOI). The masses of each sample were measured after 105 ℃ and 900 ℃. 6.0000 ± 0.0005 grams of lithium metaborate (49.75% Li2B4O7, 49.75% LiBO2, and 0.5% LiBr) was mixed with 0.6000 ± 0.0005 grams of each sample and fused to produce a glass disc using a Claisse M4 fluxer. The major oxide concentrations were measured by WD-XRFS using a PANalytical Axios mAX spectrometer at National Taiwan Normal University in Taipei. The measured major elemental data of United States Geological Survey standard reference materials BIR-1 and SDC-1 are reported in table S5.
4.5. Whole Rock Trace Elements
Trace elements were measured using an Agilent 7500cx inductively coupled plasma mass spectrometer (ICP-MS) at the Institute of Earth Sciences, Academia Sinica, Taipei. Approximately 40 mg of fused glass bead from each sample was dissolved using super-pure HF and HNO3 (1:1) mixture in screw-top Teflon beakers for > 12 h at ~140 °C, followed by evaporation to dryness, and refluxed in 2 ml HNO3 (1:2) for > 12 h at ~140 °C. Then samples were diluted using 2% HNO3 and 10 ppb Rh and Bi spike was added for the internal standard. The spiked solution was diluted with 2% HNO3 to a sample/solution weight ratio of 1:1500. United States Geological Survey standard reference materials analyzed for trace elements are BCR-2, BHVO-2, and DNC-1. The accuracy of the measured standard reference material is better than ± 5 % for all elements (table S5).
4.6. Thermal Ionization Mass Spectrometry
Strontium isotopes were measured at two different laboratories. Samples NI-002 and NI-003 were measured at Activation Laboratories, Ancaster, Ontario. The samples were dissolved using HF+HNO3 and Sr was separated following the procedures of Creaser et al. (2004) and Holmden et al. (1997). Isotopic analysis was carried out using a multi-collector inductively coupled plasma mass spectrometer (MC-ICP-MS). All analyses are presented relative to a value of 0.710245 for the SRM987 Sr isotopic standard. The remaining samples (NI-004, NI-009, NI-010, NI-016) were analyzed at Academia Sinica, Institute of Earth Sciences, Taipei, Taiwan using a Finnigan MAT-262 thermal ionization mass spectrometer (TIMS). Each sample was weighed (75–100 mg) and loaded into a Teflon beaker, dissolved by a mixture of HF and HNO3 (50 drops of each acid), and heated on a hotplate (~100 ℃) for 48 hours. The solution was dried and another 2 mL 6N HCl was added to redissolve the samples. This step was done twice in order to ensure complete dissolution. After drying, another 2 ml 1N HCl was added to the beaker and followed by centrifuging for 10 minutes. The supernatant was transferred to a new beaker. This step was repeated until all of the samples were digested. The samples were then added to columns (2.5 ml 100–200 mesh Bio-Rad AG 50W-X8 cation exchange resin) to separate Sr and rare earth elements (REEs). The columns were equilibrated with 2N HCl before loading the samples. After the sample solution was loaded into the column, 3 mL 2N HCl was added to the beaker to wash out the remaining sample and then loaded into the column, followed by 18 mL 2N HCl to rinse. Strontium was collected following the addition of 12 mL 2N HCl in a new beaker. Another 3 mL 2 N HCl was added and discarded. The rare earth elements were collected following 13 mL 6N HCl in a new beaker. The solutions of Sr and REE were dried and 1 mL 2N HCl was added to the Sr beaker and 0.1mL milliQ water to the REE beaker.
The strontium column (1 mL 100–200 mesh Bio-Rad AG 50W-X8 resin) was used to further purify the Sr. The columns were first equilibrated using 2N HCl. The Sr solution separated from the initial column was loaded into the Sr column. One mL of 2N HCl was added into the beaker to wash out the remaining sample and loaded into the column. The beaker-wash out step was repeated twice. Another 4 mL 2N HCl was added into the column. Strontium was collected following 10 mL 2N HCl and dried after the collection. One drop each of HCl and HNO3 was added to the dried sample and then repeated three times. Rare earth elements were separated using polyethylene columns with a 5 ml resin bed of AG 50W-X8, 200–400 mesh. Neodymium was separated from other REEs using an Ln resin as a cation exchange medium. The samples were loaded on to Re filaments with one drop of H3PO4 and then dried with a 0.8–1.0 mA current. The 143Nd/144Nd ratios were normalized to 146Nd/144Nd = 0.7219 and 87Sr/86Sr ratios to 86Sr/88Sr = 0.1194. The Sr isotopic ratios were measured using a Finnigan MAT-262 thermal ionization mass spectrometer (TIMS) whereas the Nd isotopic ratios were measured using a Finnigan Triton TIMS in the Mass Spectrometry Laboratory, Institute of Earth Sciences, Academia Sinica, Taipei. The 2σm values for all samples are less than or equal to 0.000028 for 87Sr/86Sr and less than or equal to 0.000007 for 143Nd/144Nd (table S6). The measured isotope ratio for JMC Nd standard is 0.511813 ± 0.000010 (2σm) and NBS987-Sr is 0.710248 ± 0.00001 (2σm).
5. Results
5.1. Mineral Chemistry
5.1.1. Olivine
Fayalite was analyzed from sample NI-003 and it has a nearly pure end-member composition with a narrow range of Fa92.4−94.5. Silica ranges from ~29.5 to ~30.8 wt.% with MnO from ~3.1 to ~3.9 wt.% and MgO from ~2.1 to ~2.9 wt.%.
5.1.2. Orthopyroxene
Orthopyroxene (ferrosilite) is not abundant (< 1 vol.%) in the North Island Complex and was analyzed from sample NI-009. The crystals have a compositional range of Wo1.5−1.8En19.7−21.3En78.9−76.9 and Mg# of 20.0 and 21.7. The concentrations of Al2O3 (0.14–0.16 wt%), TiO2 (< 0.1 wt%) and CaO (≤ 0.7 wt%) are low. It is possible that the ferrosilite is a secondary mineral as the major oxide sum totals tend to be low (96–98 wt%).
5.1.3. Clinopyroxene
The clinopyroxene classify as diopside-hedenbergite within the pyroxene quadrilateral and have compositions of Wo44.8-50.0En13.9-32.8Fs20.6-40.6. The minor elements such as TiO2 (0.14−0.64 wt.%), Al2O3 (0.65−1.04 wt.%), MnO (0.44–1.11 wt.%), and Na2O (0.34−0.70 wt.%) are similar across all samples. The crystals in the diorites are MgO-rich (Wo45.5-50.0En22.8-32.8Fs20.6-28.0) compared to those from the syenites (Wo44.8-47.3En13.9-23.8Fs30.8-40.6).
5.1.4. Amphibole
The amphiboles from the North Island syenites are calcic and classify (CaB ≥ 1.5; (Na+K)A ≥ 0.50; Ti < 0.5; Mg/(Mg+Fe2+) < 0.5) as hastingsite-ferropargasite (Leake et al., 1997). The cation mineral formulae were calculated using PROBE-AMPH of Tindle and Webb (1994). The TiO2 (1.51–4.27 wt%), Al2O3 (6.70–10.08 wt%), TFeO (26.51–31.14 wt%), MgO (2.75–4.30 wt%), CaO (9.94–10.97 wt%), Na2O (2.05–3.12 wt%), and K2O (0.79–1.59 wt%) concentrations are variable. However, the amphiboles within the syenites from eastern (NI-007) North Island are generally more magnesian than those from western (NI-009, NI-014, NI-016) North Island.
5.1.5. Biotite
The biotite crystals have magmatic compositions (Nachit et al., 2005) and show compositional variability from higher MgO to lower MgO across different rock types. The diorites have biotite with Fe/Fe+Mg values of 0.64 to 0.75 whereas the biotites from the syenites have values of 0.79 to 0.97. Furthermore, the TiO2 (TiO2 = 4.97–7.09 wt.%) concentrations of the biotites from the diorites are generally higher than the biotites (TiO2 = 2.33–5.79 wt.%) from the syenites. The Al2O3 (diorite = 12.63–13.93 wt.%; syenite = 9.88–13.11 wt.%) and K2O (diorite = 8.67−9.75 wt.%; syenite = 7.62−9.38 wt.%) concentrations are similar between the two rock types. The biotite mineral formulae were calculated using the method of Tindle and Webb (1990).
5.1.6. Feldspar
Alkali feldspars are the most abundant phenocrysts in the syenites whereas plagioclase feldspar is more common in the diorites. The diorites contain sanidine-anorthoclase (An2.0−6.7Ab28.2−61.3Or32.0−69.0) and andesine-oligoclase (An8.8−29.2Ab68.1−89.4Or1.8−5.0). The syenites are composed of perthitic feldspar with near end-member albite (Ab > 90) and orthoclase (Or > 90) compositions and anorthoclase-sanidine (An1.2−6.1Ab31.4−76.5Or19.6−67.2).
5.1.7. Titanite
Titanite was analyzed in sample NI-014 and the mineral formulae were calculated on the basis of four Si. The sum totals range from 94.32 wt.% to 98.23 wt.% suggesting there is an appreciable amount of volatile (OH-, Cl-, F-) and/or trace elements in the crystals. The total iron content (2.22–3.32 wt.%), as expressed as Fe2O3, TiO2 (34.08–36.24 wt.%), and CaO (26.13–27.95 wt.%) do not vary significantly.
5.1.8. Ilmenite
The total FeO contents range from 41.38–48.68 wt.% whereas TiO2 ranges from 49.18–53.82 wt.%. The ferrous and ferric iron was calculated by charge balance and all samples have Fe3+/Fe2+ ratios of ≤ 0.11. The MnO concentration ranges from 1.79–6.37 wt.% whereas the MgO is ≤ 0.25 wt.%. The ilmenite crystals from each sample are compositionally similar.
5.1.9. Magnetite
Magnetite is less abundant than ilmenite in the rocks and has significantly more chemical variability than the ilmenite. The total FeOt contents range from 72.76–92.50 wt% whereas TiO2 ranges from 0.66–17.70 wt%. Ferric and ferrous iron are calculated by charge balance and all samples have Fe3+/Fe2+ ratios from 0.63 to 1.82. The MnO concentration ranges from below detection limit to 1.91 wt%. There is no observable difference between the magnetites from across the samples.
5.2. Zircon U-Pb Geochronology
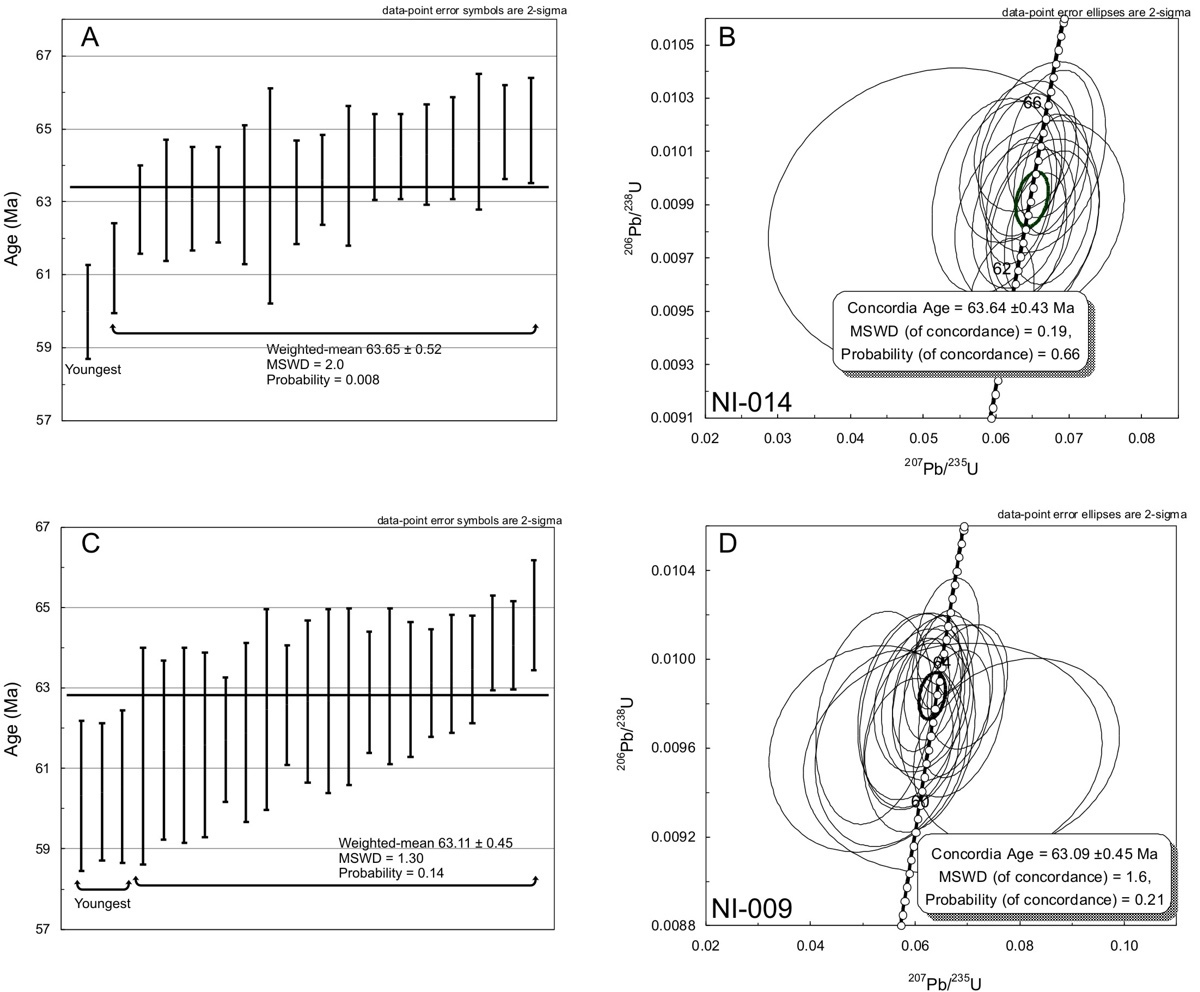
Twenty-one (24 spots) individual zircon crystals were analyzed from sample NI-014 (table S3). The zircons range in length from ~100 µm to ~500 µm and have euhedral to subhedral shapes, but many crystals are fragmented (fig. S1). Some zircons exhibit oscillatory zoning (e.g., 1, 9, 12, 14, 15, 16-17, 19, 20–21, 23–24) and lack core-rim structures, but there are a few crystals that appear to have irregular core-rim structure (e.g., 2, 6, 8, 10, 11, 18) whereas others (e.g., 3, 4, 5, 7, 13, 22) have textures similar to post-magmatic recrystallized zircon (cf., Corfu et al., 2003). The targeted spot locations were regions without structure and have oscillatory zoning. The total range of the 238U/206Pb ages is from 60.0 ± 1.3 Ma to 95.9 ± 6.6 Ma. Measured uncertainties are reported at the 2σ level for each spot analysis (table S3). The Th/U ratios range from 0.05 to 2.15 with the oldest zircon having the lowest value. Our results show that there are three age populations of zircons. The youngest zircon has a 238U/206Pb age of 60.0 ± 1.3 Ma which is anomalously young relative to the main population of zircons (fig. 5A). The main population (17 zircons) has 238U/206Pb ages from 61.2 ± 1.2 Ma to 65.0 ± 1.4 Ma. The oldest zircons have ages from 68.0 ± 1.1 Ma to 95.9 ± 6.6 Ma which we consider to be inherited, in part due to their post-magmatic recrystallization textures, and are not included in the crystallization age calculation. If all zircons except those considered to be inherited are used to calculate the weighted-mean age (63.40 ± 0.67 Ma) then the resultant age has a high MSWD (3.7) and a probability of confidence of 0. Furthermore, a Concordia age cannot be determined. If only the zircons of the main population are considered than the weighted-mean 238U/206Pb age 63.65 ± 0.52 Ma (MSWD = 2.0). The Concordia age of the main population is 63.64 ± 0.43 Ma (MSWD = 0.19) (fig. 5B). Consequently, we consider the main population zircons to be representative of the crystallization age of the rock.
Twenty-four zircon crystals were analyzed from sample NI-009 (table S3). The zircons range in length from ~75 µm to ~150 µm and have euhedral to subhedral shapes (fig. S2). The zircons exhibit oscillatory zoning and very few have core-rim structures. The range of the 238U/206Pb ages is from 60.3 ± 1.9 Ma to 68.8 ± 1.2 Ma. Measured uncertainties are reported at the 2σ level for each spot analysis (table S3). The Th/U ratios range from 0.41 to 1.93 and within the range of igneous zircons (Hoskin & Schaltegger, 2003). Similar to sample NI-014, three populations are identified. The three youngest zircons are 60.3 ± 1.9 Ma, 60.5 ± 1.9 Ma, and 60.4 ± 1.7 Ma and are similar to the youngest zircon from sample NI-014. The main population (20 zircons) range in age from 61.3 ± 2.7 Ma to 64.2 ± 1.4 Ma whereas the oldest zircon is considered to be inherited (68.8 ± 1.2 Ma). If all zircons except those considered to be inherited are used to calculate the weighted-mean age (62.92 ± 0.52 Ma) then the resultant age has a high MSWD (2.1), low probability of confidence of 0.002, and a Concordia age cannot be calculated (fig. 5C). In comparison, the weighted-mean 238U/206Pb age of the main population is 63.11 ± 0.45 (MSWD = 1.3) and the Concordia age is 63.09 ± 0.45 Ma (MSWD = 1.76) (fig. 5D). We consider the main population zircon group to be representative of the crystallization age of the rock.
The crystallization ages (63.65 ± 0.52 Ma and 63.11 ± 0.45) of NI-014 and NI-009 and their uncertainty fall within the range of the zircon U-Pb ID-TIMS ages (63.28 ± 0.08, 63.31 ± 0.11, 63.20 ± 0.12 Ma, 63.24 ± 0.14) reported by Ganerød et al. (2011) for North Island and are older than the ages (60.6 ± 0.6 Ma to 61.0 ± 0.8 Ma) reported by Shellnutt et al. (2017). The discrepancy between the younger and older spot ages could be related to the methods used (i.e., LA-ICP-MS vs. LA-ICP-MS/MS). However, the identification of younger zircons in this study suggests that the magmas may have crystallized over a prolonged period of time (i.e., 60–63 Ma).
5.3. Zircon Hf Isotopes
Hafnium isotopes were analyzed on the same spot locations as reported for the U-Pb ages of samples NI-002, NI-004, NI-010, and NI-017 by Shellnutt et al. (2017). Not all zircons were analyzed for Hf isotopes because of the limited amount of material remaining after the initial session of ablation for geochronology. The initial ratios and εHf(t) values of the zircons were calculated on the basis of their individual U-Pb age. The complete dataset is in supplementary table S4 and the depleted mantle evolution and crust evolution curves in figure 6 are defined using present-day 176Hf/177Hf = 0.28325 and 176Lu/177Hf = 0.0384 (Griffin et al., 2000).
Nine zircons from NI-002 (diorite) yielded initial 176Hf/177Hf(t) ratios from 0.282855 to 0.282970 with εHf(t) values ranging from +4.3 to +8.4. The TDM1 ages range from 402 Ma to 558 Ma whereas the TDM2 ages range from 598 Ma to 859 Ma. Five zircons from sample NI-004 (syenite) have initial 176Hf/177Hf(t) ratios that range from 0.282859 to 0.282903 with εHf(t) values ranging from +4.5 to +6.0. The TDM1 ages range from 505 Ma to 569 Ma whereas the TDM2 ages range from 752 Ma to 849 Ma. Sample NI-010 (syenite) has the most analyses (19) and yielded initial 176Hf/177Hf(t) ratios from 0.282792 to 0.282905 with εHf(t) values ranging from +2.1 to +6.1. The TDM1 ages range from 489 Ma to 655 Ma whereas the TDM2 ages range from 746 Ma to 1002 Ma. Six zircons from NI-017 (syenite) yielded initial 176Hf/177Hf(t) ratios from 0.282797 to 0.282913 with εHf(t) values ranging from +2.2 to +6.3. The TDM1 ages range from 487 Ma to 659 Ma whereas the TDM2 ages range from 729 Ma to 992 Ma.
5.4. Major and Trace Elemental Compositions
The rocks collected for this study include syenite (SiO2 = 61–65 wt%), one microsyenite (SiO2 = ~70 wt%) rock, and two diorites (SiO2 ≈ 57 wt%) (fig. 7A). The alumina saturation index values show that all but one of the syenitic rocks are metaluminous (mol. Al2O3/mol. CaO+Na2O+K2O < 1; mol. Na2O+K2O/Al2O3 < 1) whereas the other is weakly (mol. Na2O+K2O/Al2O3 = 1.01) peralkaline (fig. 7B). Most rocks are quartz, diopside, and hypersthene normative whereas two are wollastonite normative instead of hypersthene normative and two are olivine and nepheline normative. The dioritic rocks are olivine, nepheline and diopside normative. The concentration of MnO (i.e., < 0.2 wt%), MgO (i.e., ≤ 0.6 wt%) and CaO (i.e., < 2.1 wt%) are low. All of the rocks have high Fe* (FeOt/MgO+FeOt ≥ 0.80) values and classify as alkalic according to the modified alkali lime index scheme of B. R. Frost et al. (2001) (fig. 7C and D). The trace elemental characterization using the criteria of Eby (1992) and Whalen and Hildebrand (2019) shows the rocks to be similar to the A-type (A1) granitoids which is consistent with the major elemental classification scheme of B. R. Frost et al. (2001) (fig. 7E and F).
All of the rocks have uniformly low concentrations of transition metals with no distinction between the syenites, diorites, and microsyenite (Sc = 13–24 ppm, V ≤ 22 ppm, Cr = 1–33 ppm, Co ≤ 9 ppm, Ni = 1–96 ppm, Cu ≤ 10 ppm, Zn = 44–128 ppm). The large ion lithophile element (LILE) concentrations are more variable (Rb = 36.3–276 ppm, Sr = 3–521 ppm, Cs = 0.3–3.4 ppm, Ba = 9–12170 ppm). Sample NI-017 has the lowest Sr and Ba concentrations and the highest concentration of Rb. Overall, the concentration of Ba in all rocks is high with most samples having ≈1300 ppm whereas NI-002 has a value of 12170 ppm (BaO = ~1.3 wt%). The high field strength elements (HFSE) are as equally variable as the LILE (Zr = 61–605 ppm, Nb = 35–230 ppm, Y = 13–57 ppm, Hf = 1.36–13.6 ppm, Ta = 1.83–16.1 ppm, Th = 1.84–40.4 ppm, U = 0.53–9.49 ppm), but the microsyenite has the highest concentrations of incompatible elements. The primitive mantle normalized incompatible patterns of the rocks, for the exception of the microsyenite, are broadly similar although there is some variability in the Rb−Ba and Hf−Zr concentrations (fig. 8A and B). All rocks have similar light rare earth element enriched chondrite normalized rare earth element patters with high LaN/SmN (4.7–13.0) and LaN/YbN (8.0–18.0) ratios (fig. 8C and D). Many of the samples have positive Eu-anomalies with Eu/Eu* values [EuN/(SmN×GdN)0.5] ranging from 0.07 (NI-017) to 8.13 (NI-002) with most samples having values of 0.97–2.60.
5.5. Sr and Nd Isotopes
Six samples were analyzed for Sr and Nd isotopes (table S6). The initial isotopic ratios of the rocks (diorite, syenite) were calculated based on their U-Pb ages from this and previous work. The initial 87Sr/86Sr ratios range from 0.704095 to 0.707533 with the dioritic sample having the highest value (0.707533) and the syenites with relatively uniform values (704095–0.704290). The initial 143Nd/144Nd ratios are similar for all rocks and range from 0.512621 to 0.512659. The corresponding εNd(t) values, using 147Sm/144Nd of 0.1967 and a CHURtoday value of 0.512638, are +1.2−+1.9 and fall within uncertainty (± 0.5 epsilon units) of the analysis (fig. 9). The depleted mantle model ages (TDM) range from 658 Ma to 709 Ma.
6. Discussion
6.1. Parental Magma of the North Island Plutonic Complex
The diorites, syenites, and microseynite of this study are ferroan, metaluminous, and alkalic according to the classification of B. R. Frost et al. (2001) and thus can be considered to be of similar character to the A-type granitoids (C. D. Frost & Frost, 2011). Furthermore, the biotite compositions from the syenites are also consistent with A-type granitoids (fig. 10A and B). The within-plate or rift-related tectonic setting of the North Island Complex is not in dispute as they intruded Proterozoic basement rocks and the emplacement age (~63 Ma) is correlated to the rift-to-drift transition of the Seychelles microcontinental block from western India (Collier et al., 2008; Devey & Stephens, 1992; Ganerød et al., 2011; Shellnutt et al., 2017). However, A-type granitoids can be subdivided on the basis of their trace elemental chemistry and mineralogy into those derived from oceanic-island basalt-like magma and those derived from crustal sources (Abdel-Rahman, 1994; Bonin, 2007; Eby, 1992; Loiselle & Wones, 1979; Shabani et al., 2003; Whalen & Hildebrand, 2019).
The high field strength trace elemental classification plots of Eby (1992) and Whalen and Hildebrand (2019) and the biotite chemistry show the North Island rocks to be of the A1-sub-type (figs. 7E, F, and 10), that is, derived from an oceanic-island basalt (OIB)-like parental magma with minimal or no contamination from crustal material (i.e., melt or fluids). The role of crustal contamination in the North Island Complex will be addressed later, but is clear from the Sr-Nd-Hf isotopic data, the low Th/NbPM (< 2) ratios, and lack of primitive mantle normalized negative Nb-Ta anomalies that the rocks were not principally derived from the continental crust. Moreover, the metaluminous bulk rock compositions and presence of Ca-rich mafic minerals (e.g., hornblende, hedenbergite) demonstrate that the North Island syenites evolved differently from the ferroan, peralkaline, and alkalic granitoids as they contain alkali mafic silicate minerals (i.e., riebeckite-arfvedsonite, aegirine). Nevertheless, we can conclude that the likely composition of the parental magma of the NIC was similar to OIB.
6.2. Magmatic Conditions of the North Island Syenite Complex
The magmatic conditions (temperature, redox state, pressure, volatile content) of the North Island syenites are constrained using mineral chemistry, mineral textures, and whole rock geochemical data. The geological relationship with the country rock is unknown as it is not exposed, but Late Paleozoic to Early Cenozoic sedimentary rocks are reported from oil exploration drill wells to the west and northeast of North Island suggesting that the rocks were emplaced within the upper crust (Plummer & Belle, 1995).
Magmatic temperatures of the North Island syenites are constrained using zircon saturation thermometry, SiO2 composition, and clinopyroxene-liquid equilibrium temperatures estimates (Boehnke et al., 2013; Duan et al., 2022; France et al., 2010; Putirka, 2008). The zircon saturation [TZr(℃)] temperatures for the rocks with M ([Na+K+2Ca]/[Al*Si]) values within the calibration range (M = 1.3 to 1.9) of the calculation show significant variability 688–872 ℃, but there are two distinct groups. Samples NI-004V, NI-011, and NI-017 have the highest temperatures (849–872 ℃) whereas samples NI-003, NI-005, NI-007, NI-008, and NI-015 are ~100℃ lower (688–737 ℃). The T-Si temperatures for all samples is 726–930 ℃ with the microsyenite sample (NI-004V) having the lowest temperature (726 ℃), the diorites having the highest temperatures (909–930 ℃), and the syenite temperatures clustering at 808–866 ℃. Although there is overlap between the TZr(℃) and T-Si temperature estimates, the T-Si results are generally higher. In comparison, the clinopyroxene-liquid equilibrium temperatures were calculated for sample NI-009 and yielded temperatures of 837 ℃, 844 ℃, and 866 ℃ and similar to the syenitic T-Si estimates and the highest TZr(℃) estimates. It is likely that clinopyroxene is the liquidus or near liquidus mineral and thus closer to the initial magmatic temperature. The Al-in-clinopyroxene thermometer (T = 93.2Al2O3 + 742 [± 40 ℃]) yielded similar temperatures as the clinopyroxene-liquid equilibrium estimates and range from 801 ℃ to 839 ℃ for all samples (France et al., 2010). The highest temperature estimates are consistent between the three different methods and we conclude that the magmatic temperature of the syenitic rocks was likely 800−900℃ and extended above 900℃ for the diorites.
The emplacement depth was calculated from the clinopyroxene-based geobarometer of Putirka (2008). The equilibrium clinopyroxene-liquid temperatures of 837 ℃, 844 ℃, and 866 ℃ correspond to pressures of 0.36 GPa, 0.33 GPa, and 0.34 GPa (table S7). Assuming 0.1 GPa = 3.7 km then the depth of clinopyroxene crystallization was 12−14 km. We also apply the titanite barometer for granitic rocks of Erdmann et al. (2019). The results yielded pressures of 0.19 to 0.27 ± 0.1 GPa (7–10 km). We think that the titanite barometer estimates are meaningful as the compositions used to calibrate the equation include ferroan and metaluminous granitic rocks and a mineral assemblage (i.e., amphibole-titanite-biotite-plagioclase-K-feldspar-quartz-magnetite±ilmenite) similar to the North Island syenites. The 0.06–0.17 GPa difference between the clinopyroxene and titanite estimates are within uncertainty of each calculation but is possible that titanite crystalized later than the clinopyroxene and that the magma crystallized over a range of pressure (0.20–0.35 GPa) before reaching neutral buoyancy in the crust.
The relative oxidation state of the syenites is estimated using biotite mineral chemistry and ilmenite and magnetite pairs (Andersen et al., 1993; Shabani et al., 2003). Equation (1) is used to calculate the oxygen fugacity estimate of Eugster and Wones (1962) for biotite. It is based upon the Fe2+-Fe3+-Mg2+ composition of biotite, and the P and T for various oxygen buffers and defined as:
LogƒO2= −AT+B+C(P−1)T
Where T is the temperature in Kelvin, P is the pressure in bar, and A, B, and C are oxygen fugacity buffer coefficients. The ƒO2 calculation is dependent upon the coefficient (A, B, and C) values (Eugster & Wones, 1962). The coefficients are chosen based on the proportions of Fe2+, Fe3+, and Mg2+ in biotite, which for this study are calculated using the program Fe23 (Nenova, 1997). The fayalite-magnetite-quartz (FMQ), Ni-NiO (NNO), and hematite-magnetite (HM) buffers are arranged in Fe2+-Fe3+-Mg2+ space and show the biotites fall along the FMQ buffer. For the redox estimates we used pressure equal to 0.3 GPa (3000 bar) and calculated the biotite temperatures using the method of Luhr et al. (1984). The results indicate that the biotite crystallized at logƒO2 values from −15.1 to −19.8 which correspond to ∆FMQ values of −0.56 to −0.95 and indicate reducing to slightly reducing conditions (table S1). Furthermore, the ilmenite, magnetite, and clinopyroxene mineral chemistry provided additional constraints on the redox conditions using QUIlF (Andersen et al., 1993). Trial values, that is those allowed to vary to find equilibrium, of temperature (1000 ℃) and initial logƒO2 values (logƒO2 = –10) were entered at a pressure of 0.3 GPa (3000 bar). The results of the calculations for samples NI-002, NI-003, NI-007, and NI-009 are in table S8. Samples NI-002 and NI-009 produce the highest temperatures (791 ℃, 733 ℃) and logƒO2 values (−15.21, −17.10) whereas NI-003 and NI-007 produced the lowest temperatures (562 ℃, 609 ℃) and logƒO2 values (−20.54, −18.53). We consider the results from NI-003 and NI-007 to be indicative of the subsolidus re-equilibration conditions rather than magmatic conditions as the temperatures are well below the expected solidus (600–650 ℃) of the syenites. However, the temperature estimates from NI-002 and NI-009 approach the estimated magmatic range (i.e., 800–900℃) of the syenites and diorites and are probably closer to their crystallization conditions. If this is the case, then the redox conditions of the magnetite-ilmenite-clinopyroxene system crystallized under moderately reducing conditions (∆FMQ = −0.72 to −1.28) and similar to the redox conditions estimated from the biotite compositions.
The volatile (e.g., OH-, F-, Cl-) concentration of the North Island syenites is poorly constrained. The presence of biotite, calcic amphibole, apatite, and titanite implies that the magma contained volatiles, but the whole rock loss on ignition values are low (0.34–0.85 wt%) and the rocks are mostly composed of hypersolvus (perthitic) alkali feldspar suggesting a relatively dry magma system (Martin & Bonin, 1976). Since magmatic carbonate and fluorine-rich minerals (e.g., bastnäsite, fluorite) were not observed in the rocks it is unlikely that the magma system was saturated in CO2, and/or F2. We can constrain the likely maximum water contents indirectly by using Rhyolite-MELTS to estimate the dry and water-rich liquidus temperatures and compare them to the clinopyroxene equilibrium, T-Si, and TZr(℃) temperatures. The dry liquidus temperatures at 0.3 GPa and ∆FMQ = −1 are 1110−1132 ℃ whereas if the LOI content is representative of the magma volatile content then liquidus temperatures under the same pressure and redox conditions is 1044−1099℃. The estimates are 150–300℃ higher than the mineral and whole rock estimates. Water saturation for the syenites at 0.3 GPa and ∆FMQ = −1 using Rhyolite-MELTS is ~6 wt%, but it was discovered that the lowest water-rich liquidus temperatures occurred at 4.5 wt% water and ranged from 885 ℃ to 969 ℃. The results suggest that the water-rich liquidus temperatures are within uncertainty of the clinopyroxene and whole rock temperature estimates indicating that the syenites may have contained up to 4.5 wt% water. If this was the case, then it is possible that the syenitic magma degassed a significant amount of water during emplacement and before significant crystallization the feldspars (cf., Shellnutt et al., 2022).
6.3. Magmatic Differentiation of the North Island Complex
The classification of the North Island syenites as an A1-sub type implies that they are the products of mafic magma differentiation (Bonin, 2007; Eby, 1992; Loiselle & Wones, 1979). The whole rock and mineral chemical data indicate that the parental magma of the North Island syenites was OIB-like and the conditions under which the syenites formed were reducing to moderately oxidizing (∆FMQ = 0 to −1) and occurred at a pressure of 0.20–0.35 GPa.
Fractional crystallization modeling was carried out using Rhyolite-MELTS version 1.0.2 (Gualda et al., 2012) and a mafic starting composition similar to a gabbro (LA97N17A) reported from North Island by Owen-Smith et al. (2013). The parental composition used for the modeling is not primitive as the MgO (wt%) concentration (3.7 wt%) and Mg# (40) are low. Therefore, it is assumed that the parental magma for the model underwent a previous period of mafic silicate (e.g., olivine, clinopyroxene, orthopyroxene) crystal fractionation as it transited through the crust. The high concentration of Sr (770 ppm) and a positive chondrite normalized Eu-anomaly (Eu/Eu* = 1.6) of the parental composition is indicative of feldspar accumulation suggesting that plagioclase may have been crystallizing at the time of emplacement, but that it did not fractionate. We think that sample LA97N17A represents an earlier magmatic stage between the primary magma composition and the diorites as the highest plagioclase An value from the gabbro (An37) is higher than the highest value (An30) from the diorite (Owen-Smith et al., 2013). It is possible that the magma was at or close to water saturation based on the Rhyolite-MELTS liquidus temperature estimates. If this was the case, then it is possible that plagioclase crystallization was initially suppressed prior to magma emplacement which subsequently enhanced feldspar accumulation (Pichavant & Macdonald, 2007; Sisson & Grove, 1993). The modeling redox and pressure conditions were set to ∆FMQ = 0 and −1 and 0.3 GPa, and the initial water content of 1.5 wt% was selected as it is within the range of continental alkalic basalts but also higher than what would be expected for a primary OIB magma (Hauri, 2002; Liu et al., 2015). The modeling composition and conditions are summarized in table S9.
The liquid evolution curves of the ∆FMQ = −1 (white dots) and ∆FMQ = 0 (black dots) models are shown as Fenner diagrams in figure 11. The complete modeling results and the crystallization assemblages can be found in online supplementary table S9. The liquid compositions are shown at 10 ℃ intervals and the starting temperature was set to 1300 °C. The liquidus temperature for the ∆FMQ = −1 model is 1200 ℃ when whitlockite crystallizes. The compositional range of the silicic system is generated from ~920 ℃ to ~800 ℃. At 800 ℃, ~83.5% of the total magma system crystallized with ~16.5% liquid remaining. The fractionated assemblage from the liquidus temperature to 800 ℃ is: whitlockite (1200–1130 ℃), apatite (1120–800 ℃), olivine (1110–850 ℃), clinopyroxene (1110−1060 ℃, 1040−1030 ℃), spinel (1090−800 ℃), plagioclase (1060−800 ℃), alkali feldspar (890−800 ℃), and biotite (850−800 ℃). The proportions relative to the total solid and compositional ranges of the fractionated minerals at 800 ℃ are: whitlockite = 0.2%, apatite = 3.4%, olivine (Fo70-25) = 11.3%, clinopyroxene (Wo56-44En30-36Fs13-20) = 14.3%, spinel (titanomagnetite) = 8.5 wt%, plagioclase (An63-25) = 43.1%, alkali feldspar (An4-2Ab37-32Or59-66) = 19.2%, and biotite = 0.2%.
The liquidus temperature for the ∆FMQ = 0 model is 1120 ℃ as spinel and apatite crystallize. The compositional range of the silicic system is generated from ~920 ℃ to ~800 ℃. At 800 ℃, ~84% of the total magma system crystallized with ~16% liquid remaining. The fractionated assemblage from the liquidus temperature to 800 ℃ is: spinel (1120−770 ℃), apatite (1120−770 ℃), clinopyroxene (1100−1040 ℃), plagioclase (1070−810 ℃), olivine (1050−940 ℃), ilmenite (940−800 ℃), orthopyroxene (930 ℃), biotite (920−800 ℃), alkali feldspar (890−800 ℃), and leucite (800 ℃). The proportions, relative to the total solid, and compositional ranges of the fractionated minerals at 800 ℃ are: spinel (titanomagnetite) = 13.6%, apatite = 3.5%, clinopyroxene (Wo48-45En37-40Fs15) = 16.6%, plagioclase (An64-21) = 43.6%, olivine (Fo70−66) = 2.7%, ilmenite = 0.5%, orthopyroxene (Mg# = 71) = 0.2%, biotite = 1.2%, alkali feldspar (An4-2Ab41-33Or55-65) = 16.9%, and leucite = 1.1%.
The models show that the liquid evolution curves can reach the bulk composition of the syenitic rocks and pass through or closely to the dioritic rocks. The ∆FMQ = −1 is the better result as the primary difference between the models is the Fe2O3t liquid evolution. The ∆FMQ = 0 model shows an earlier and greater reduction in Fe2O3t and misses the intermediate compositions and the majority of the syenitic rocks. For both models, the bulk Na2O concentration is slightly higher than that of the rocks, but the modeled total alkalis (Na2O+K2O) and K2O concentration match well and the modeled alkali feldspar compositions (An4-2Ab37-32Or59-66) overlap with the majority of the measured alkali feldspar compositions (An4-1Ab67-31Or29-67) in the rocks. It is possible that the whole rock compositions are not exclusively a consequence of crystal fractionation and may involve crystal accumulation as the Eu/Eu* values of many syenites are much greater than unity and the Ba concentrations (449–2071 ppm) can be high or very high (12170 ppm).
To examine the trace element evolution (Eu, Sm, and Gd) in the system we used MELTS (Smith & Asimow, 2005) and the same conditions and starting composition (LA97N-17A: Sm = 7.3 ppm; Eu = 3.9 ppm; Gd = 7.5 ppm) as the Rhyolite-MELTS models. The results are shown in figure 12 and it is clear that the expected compositional evolution curve diverges from the data at higher SiO2 contents (table S10). The majority of samples show higher Eu/Eu* values with a minority that fall along the model curve. The implication is that the high Eu/Eu* values of the syenitic rocks are not expected and therefore, could be a consequence of feldspar accumulation. It is possible that crystal accumulation occurred fairly late in the crystallization history as the model shows that the divergence in Eu/Eu* values does not occur until the liquid SiO2 composition reaches ≥ 62 wt.%, but the dioritic rocks also have Eu/Eu* values greater than unity. Furthermore, the syenite samples located in the eastern portion of the island (Bernica, Congoment) tend to have higher Eu/Eu* values (1.80–3.20) compared to those from the western portion (Grand’Anse, Mt. Des Cèdres) of the island (Eu/Eu* = 0.44−2.03). The compositional differences correlate with the rock textures as the rocks with the lowest Eu/Eu* values (0.07–1.11) are medium grained (NI-011, -012, -013, -017). It could imply that the medium grained rocks are closer to the chilled margin and/or liquid composition of the intrusion. In spite of the uncertainty in the precise parental magma composition, we conclude that chemical differentiation of the North Island complex was primarily controlled by crystal fractionation under reducing conditions (∆FMQ = −1) in the upper crust and that crystal accumulation of feldspar likely occurred during the formation of the gabbro, diorite, and most of the syenites. It is likely that the NIC was a closed or near-closed magma system.
6.4. Magma Source and Crustal Contamination
The parental magma of the North Island Complex was likely mafic and derived from a mantle source, but was unlikely to be from a depleted mantle source as mid-ocean ridge basalt tends to be tholeiitic rather than alkaline (or transitional) and has lower TiO2 (2.1−1.1 wt%) contents (Gale et al., 2013). The parental composition used for the modeling is a ‘best guess’ based on available data. The whole rock Sr-Nd-Hf isotopic values (ISr = 0.70387−0.70408; εNd(t) = +1.4–+3.5; εHf(t) = +0.8), low Th/NbPM (0.5–0.7) and high Nb/U (45–70) ratios, lack of a negative primitive mantle normalized Nb-Ta anomalies in LA97N17A and other mafic rocks of North Island suggest that they were derived from an oceanic hotspot source (Dickin et al., 1986; Owen-Smith et al., 2013). The isotopic and trace element ratios of the syenitic and dioritic rocks of North Island are within the range of the mafic rocks (ISr = 0.70364–0.70429; εNd(t) = +1.4–+3.8; εHf(t) = +2.1–+8.4, Th/NbPM = 0.3–1.6; high Nb/U = 28–109). The Tb/YbPM values of the North Island mafic rocks are 1.8 and 1.9 which are lower than the OIB composition of Sun and McDonough (1989) and places them within the range of basalts derived from the garnet-spinel transition zone (Wang et al., 2002).
An oceanic island/hotspot source of the parental magma is plausible as rifting of the Seychelles microcontinent from India is contemporaneous with the eruption of the Deccan Traps at ~65 Ma (Basu et al., 1993; Pande, 2002; Schoene et al., 2015). The Deccan Traps are thought to be a consequence of melting of the Réunion hotspot although there is debate and alternative mantle sources are proposed (Glišović & Forte, 2017; Self et al., 2022). Nevertheless, geochemical evidence for crustal contamination from materials derived from the ancient basement of the Seychelles microcontinent in the North Island Complex is limited to non-existent and did not play a significant role in the genesis of the rocks. The only evidence of contamination in the North Island syenites is the presence of older zircons. Five zircons in sample NI-014 have 238U/206Pb ages ranging from 68 ± 1 Ma to 73 ± 1 Ma whereas a single zircon from sample NI-003 was reported by Shellnutt et al. (2017) to have a 238U/206Pb age of 68 ± 4 Ma. These zircons are anomalous relative to the main zircon populations of the North Island rocks but are contemporaneous with ages reported from Deccan Traps-related rocks in western India and Pakistan and basaltic rocks reported from the western bank (Owen Bank A-1 and Seagull Shoals-1 wells) of the Seychelles microcontinent (Basu et al., 1993; Dongre et al., 2022; Kerr et al., 2010; Mahoney et al., 2002; Plummer, 1995). The Deccan-related mineral and whole rock 40Ar/39Ar ages range from 68.17 ± 1 Ma to 73.4 ± 2.0 Ma and are considered to be indicative of pre-flood basalt volcanic activity associated with the Réunion hotspot (Armitage et al., 2011; Kerr et al., 2010). The inherited zircons suggest that pre-flood basalt magmatic rocks may exist within the Seychelles microcontinent and that pre-Deccan magmatism should be present in the coastal regions of Gujarat and Maharashtra. The Sr-Nd isotopes of the ~70 Ma basaltic rocks (87Sr/86Sri = 0.70382–0.70728; εNd(t) = +2.6–+4.6) reported by Mahoney et al. (2002) and Kerr et al. (2010) from Pakistan partially overlap with the North Island rocks. The reported basaltic rocks from Owen Bank and Seagull Shoals have whole rock K-Ar ages ranging from 70.9 ± 1.1 Ma to 77.6 ± 1.0 Ma, but little else is known (Plummer, 1995). The Sr-Nd isotopes (87Sr/86Sri = 0.70211–0.70309; εNd(t) = +0.7–+3.2) of the ~750 Ma granites from the Mahé Group are also similar to the North Island rocks (Ashwal et al., 2002; Shellnutt et al., 2020). Thus, even if contamination of the North Island syenites by the ~70 Ma or ~750 Ma rocks occurred, it would be very difficult to confirm from an isotopic perspective. The single oldest inherited zircon reported from this study (95.9 ± 6.6 Ma) is contemporaneous with volcanism associated with the Marion hotspot and the rifting of Madagascar from India and also basalt (84 ± 16 Ma) dredged from the Amirante ridge, ~600 km to the southwest of North Island (Plummer, 1995; Storey et al., 1995; Torsvik et al., 1998).
6.5. Implications for the Tectonomagmatic Evolution of the North Island Complex
The data and modeling presented in this study suggest that the parental magma of the North Island Complex was derived from the Réunion hotspot and emplaced into the continental lithosphere of the western India plate at ~63 Ma. Initial crystallization of primitive mafic minerals likely occurred as the parental magma passed through the lower crust before reaching the upper crust. The resultant magma reached neutral buoyancy in the upper crust and continued to differentiate by fractional crystallization and feldspar accumulation. Contamination by crustal material was very limited or had a negligible influence on the evolution of the North Island Complex. Emplacement and differentiation of the parental magma occurred within a continental rift setting that eventually led to the separation of the Seychelles microcontinent from India and sea-floor spreading at the Carlsberg Ridge (Collier et al., 2008; Shellnutt et al., 2017).
The magmatic history of the North Island Complex is further revealed by the whole rock compositions and thermodynamic modeling. The syenitic rocks (NI-003 to NI-008) from the eastern part of North Island are chemically distinct from those of the western part (NI-009 to NI-017). For example, the eastern rocks tend to have lower incompatible element concentrations than the western rocks (fig. 13). Moreover, the eastern syenites tend to have higher Sr concentrations and Eu/Eu* values than the western syenites. It is likely that the chemical differences are related to fractional crystallization as Ga, Rb, Y, Zr, Nb, REEs (except Eu), Hf, Ta, Th, and U are more incompatible and prefer to stay in the magma than partition into a mineral. Furthermore, the presence of the dioritic rocks in the east is unlikely to be happenstance as they represent the intermediate stage of the magma system. The low Eu/Eu* values (0.44–0.97) of samples NI-011 and NI-012 are consistent with expectations of the MELTS trace element model in figure 12 and were collected near the peak of Mt. Des Cèdres, the highest elevation of North Island. Therefore, it is possible that the elevated western portion of North Island represents the upper part of the magma chamber and is composed mostly of rocks derived from the fractionated magma with comparatively less accumulated feldspar (fig. 14).
Another implication of the study is for the general petrogenesis of metaluminous, ferroan, and alkalic A-type granites. C. D. Frost & B. R. Frost (C. D. Frost & Frost, 1997, 2011) suggest that the difference between the development of metaluminous and peralkaline quartz-bearing, ferroan, alkalic granitic rocks is the parental magma composition. For example, metaluminous ferroan, alkalic rocks are likely derived by differentiation of tholeiitic basalt whereas the peralkaline ferroan, alkalic rocks are derived by differentiation of transitional to alkali basalt. Moreover, pressure, relative oxidation state, and volatile content play important roles as to whether peralkaline or metaluminous silicic rocks are generated by mafic magma differentiation (C. D. Frost & Frost, 2011; Macdonald, 2012). In the case of the North Island Complex, it is unlikely that the parental magma was tholeiitic. The total alkali (Na2O+K2O wt%) content of the syenitic rocks of this study exceeds 11 wt%, the contemporaneous basaltic rocks reported from North Island and Silhouette are alkaline, and the most calcic plagioclase in the North Island gabbroic rocks is An37 which is common for the late crystallization stages of alkali basalt (Dickin et al., 1986; Greenough et al., 2005; Owen-Smith et al., 2013). Yet, the North Island Complex is metaluminous rather than peralkaline. The likely reason for the metaluminous character of the North Island syenites is their depth of differentiation rather than the parental composition or redox condition (e.g., Shellnutt, 2021).
The models presented in this study show that the initial feldspars crystallize after clinopyroxene and at 1060–1070 ℃ with a composition of An62.9−63.5 whereas changing the model pressure to 0.1 GPa but maintaining the redox conditions and water content yielded feldspar compositions of An70.8−71.8 at 1060–1070 ℃. The decrease in pressure causes a significant change in the An content (7–9 An units) of the feldspars. Furthermore, the duration and timing of clinopyroxene exerts an important influence. In our models, clinopyroxene crystallizes over a short duration (model 1 = 1110–1060 °C, 1040–1030 °C; model 2 = 1100–1040 °C) and only overlaps with plagioclase (model 1 = 1060–1030 °C; model 2 = 1070–1040 °C) for 30–40 °C. The early and ‘short’ period of clinopyroxene crystallization reduces the amount of available Ca in the magma without reducing Al. Consequently, the initial plagioclase will crystallize from a magma that is relatively depleted in Ca but enriched in Al. Since sodic plagioclase requires less Al to crystallize, due to the charge balance between Na+ and AlSi3O8-, the ASI value will be relatively higher during magma crystallization. At lower pressure (0.1 GPa) the interval between initial plagioclase crystallization and clinopyroxene crystallization is 20 °C. Thus, the shorter interval between plagioclase and clinopyroxene crystallization cannot reduce as much Ca in the magma before the onset of plagioclase crystallization and the reduction of Al in the magma. Since calcic plagioclase requires more Al to crystallized due to the charge balance between Ca2+ and Al2Si2O82-, the ASI value will be relatively lower during crystallization. Water acts to suppress the crystallization feldspar (Pichavant & Macdonald, 2007; Sisson & Grove, 1993) and thus the water content of the original model (H2O = 1.5 wt%) will delay the crystallization of feldspar compared to models with lower water content. The higher water content and pressure will cause the crystallization of feldspar after clinopyroxene and at lower An values compared to low pressure and low water conditions. Models with similar redox conditions but lower water contents (H2O = 0.5 wt%) and different pressures (0.1 GPa vs. 0.3 GPa) crystallize feldspar at higher temperatures (1100–1130 ℃), often before clinopyroxene, and yielded compositions that are different by only 2–5 An units rather 7−9 units at higher water contents. The lower water contents allow for earlier crystallization of relatively calcic feldspar as opposed to the higher water models. The implication of our modeling is that the residual magma will have higher alumina and thus evolve to metaluminous compositions rather than peralkaline compositions.
7. Conclusions
The North Island syenite complex was emplaced during the Early Paleogene (~63 Ma) and contemporaneous with the eruption of the youngest Deccan Traps. Magmatism was temporally associated with the rifting of the Seychelles microcontinent from the western margin of Peninsular India that was likely facilitated wholly or in part by the passage of the Indian plate over the Réunion hotspot. The mineral chemistry indicates that the syenitic magmas crystallized under reducing (∆FMQ = −1) redox conditions at pressure typical of the upper crust (0.19 to 0.36 GPa). Whole rock and mineral equilibrium temperature estimates of the syenites consistently yielded maximum temperatures of 800–900 ℃. Petrological modeling under the constraints determined by the mineral chemistry indicates that the North Island syenites were derived by hydrous fractional crystallization of an alkaline mafic parental magma. Feldspar accumulation likely occurred throughout the mafic-silicic crystallization history of the complex and is responsible for the positive chondrite normalized Eu-anomalies in some of the rocks. The syenites most affected by feldspar accumulation are located at Congoment and Bernica suggesting the magma system may have evolved from east to west (present position) in an upward trajectory and that the western most rocks are closer to the magma composition. Crustal contamination of the syenites by materials (melts, fluids) derived from the ancient Seychelles microcontinent basement could not be determined, although slightly order inherited zircons (68–73 Ma, ~95 Ma) were identified and testify to the existence of an older pre-flood basalt period of magmatism in the region prior to rifting. The dioritic rocks likely represent an intermediate stage of magma evolution from the original parental magma to the final syenitic rocks. The results of this study show that crystallization pressure can exert a significant influence on whether a ferroan A-type granitoid will be peralkaline or metaluminous.
Acknowledgements
We are grateful to Mark Brandon, Trishya Owen-Smith, and Claire Bucholz for their constructive comments that helped to improve this manuscript. We thank and Javier Cotin, Elliott Mokhobo and the owners of North Island resort for their generous support for field work on North Island. Carol Cheung, Cynthia Tsai, and Robert Hsieh are thanked for their laboratory assistance. Funding support was provided by National Science and Technology Council (Taiwan) grant 112-2116-M-003-005-MY2 to JGS.
Author contributions
JGS conceived of the study, collected samples, processed the data, and wrote the manuscript. TYL contributed to the writing of the manuscript. YI analyzed the mineral chemistry and contributed to the writing. HYL provide analytical expertise for the Hf isotopes and contributed the writing. CTP processed the biotite data. KS developed the in situ geochronology method and contributed to the writing.
Data availability
All data for this study are available at https://doi.org/10.17632/8mkngdftfp.2
Editor: Mark T. Brandon, Associate Editor: Claire Bucholz

_location_of_the_seychelles_microcontinent_and_(b)_regional_geological_map_of_the_inner.jpeg)




_primitive_mantle_normalized_incompatible_element_and_(b)_chondrite_normalized_plots_of.jpeg)

_classification_of_biotite_using_the_feo-mgo-al_2_o_3__discrimination_of_abdel-rahman_.jpeg)
_rhyolite-melts_fractional_crystallization_of_the.jpeg)
_and_trace_elemental_(sm__eu__gd)_modeling_using_the_s.jpeg)


_location_of_the_seychelles_microcontinent_and_(b)_regional_geological_map_of_the_inner.jpeg)





_primitive_mantle_normalized_incompatible_element_and_(b)_chondrite_normalized_plots_of.jpeg)

_classification_of_biotite_using_the_feo-mgo-al_2_o_3__discrimination_of_abdel-rahman_.jpeg)
_rhyolite-melts_fractional_crystallization_of_the.jpeg)
_and_trace_elemental_(sm__eu__gd)_modeling_using_the_s.jpeg)

