1. Introduction
The boron isotope values (δ11B) of bulk carbonate sediments have been used to track changes in marine pH and atmospheric pCO2 during critical events in Earth’s history such as the termination of Snowball Earth and the Permian-Triassic mass extinction (Clarkson et al., 2015; Kasemann et al., 2005, 2010; Ohnemueller et al., 2014). Carbonate δ11B values may also be able to provide insights into microenvironments under which carbonates precipitated. For instance, calcifying algae have anomalously positive δ11B values (Donald et al., 2017; Zhang et al., 2017). Using carbonate δ11B values as environmental proxies in ancient carbonates rests on the assumption that no significant B isotopic resetting during diagenesis—which may occur when carbonates undergo recrystallization (no change in mineral phases) or neomorphism (transformation of metastable high-Mg calcite or aragonite to low-Mg calcite or dolomite) (Knauth & Kennedy, 2009; Land, 1986). Carbonate diagenesis can be broadly subdivided into meteoric and mixing zone diagenesis, marine burial diagenesis, and deep burial diagenesis based on the composition of the diagenetic fluid and the depth of burial (e.g., Marshall, 1992; Oehlert & Swart, 2014; M. Y. Zhao, Planavsky, et al., 2020; M. Y. Zhao & Zheng, 2017). Platform carbonates altered by meteoric diagenesis on the Great Bahama Bank record significant reductions in both δ11B and B/Ca values (Stewart et al., 2015) but the influence of marine burial diagenesis on shallow-water carbonate δ11B beyond the zone of meteoric alteration remains underexplored.
The basis for boron as a pH proxy is that the relative distribution of the two aqueous boron species, boric acid B(OH)3 and the borate ion B(OH)4-, is a function of pH and there is a large isotopic fractionation between these two species. The boron isotopic fractionation between B(OH)3 and B(OH)4- is ~26 ‰ at 25 oC in seawater, with light 10B preferentially incorporated into B(OH)4- (Klochko et al., 2006; Nir et al., 2015). Borate ions are, in turn, preferentially incorporated into carbonate crystal lattices. Therefore, the B isotopic fractionation between carbonate and seawater is a function of pH. Consequently, the B isotopic composition of carbonates can be used to calculate the pH of seawater as long as the B isotopic composition of seawater is known.
Marine burial diagenesis in carbonate platforms is usually characterized by the transformation of metastable carbonates such as aragonite and high-Mg calcite to stable low-Mg calcite and dolomite (e.g., Malone et al., 2001; Melim et al., 2002; M. Y. Zhao, Planavsky, et al., 2020). On the other hand, the main pathway for marine burial diagenesis in deep-sea carbonates is the recrystallization of calcite with varying Mg contents (e.g., Brown & Elderfield, 1996; Fantle et al., 2010; Swart & Eberli, 2005). Regardless of the setting, the interaction between primary carbonate and marine porewater may significantly alter carbonate composition (e.g., Fantle et al., 2010; Higgins et al., 2018), even if the composition of marine porewater is similar to that of seawater. Many studies indicate that marine burial diagenesis significantly impacts both elemental (e.g., Mg/Ca and Sr/Ca) and isotopic records (e.g., δ13C, δ18O, δ44Ca, δ238U, and δ7Li) of marine carbonates (e.g., Brown & Elderfield, 1996; Chen et al., 2018; Dellinger et al., 2020; Fantle et al., 2010; Higgins et al., 2018; Swart & Eberli, 2005; M. Y. Zhao & Zheng, 2014), although a recent study suggests that it does not have a big impact on the Ce anomaly of carbonates (Liu et al., 2019). In contrast, the effects of marine burial diagenesis on the B isotope signatures of shallow-water carbonates remain comparatively understudied.
A few studies have investigated B isotopic variation in deep-sea carbonates during marine burial diagenesis (Edgar et al., 2015; Spivack et al., 1993; Spivack & You, 1997). These studies have revealed that the extent of alteration of δ11B in deep-sea carbonates is site-specific, which could be related to the original mineral composition and/or porewater conditions. For example, the recrystallization of bulk carbonates at ODP Site 851 corresponds with a drop in δ11B (Spivack & You, 1997). However, these changes were not observed in other deep-sea sites characterized chiefly by foraminiferal calcite (ODP Sites 803 and 865, TDP Site 18, Edgar et al., 2015; Spivack et al., 1993). While these studies are useful for interpreting δ11B in deep-sea marine sedimentary archives, these sites are likely poor analogues for marine carbonate platforms. Application of the δ11B pH and pCO2 proxy to ancient rock records, therefore, will benefit from a better understanding of B isotope behavior in modern carbonate platforms during marine burial diagenesis.
Here we provide δ11B and B/Ca measurements from an extensively studied carbonate core (ODP Leg 166 Site 1007) collected near the Great Bahama Bank to understand the influence of marine burial diagenesis on the B/Ca and δ11B values of platform bulk carbonate sediments.
2. Materials and Methods
2.1. Study site and samples
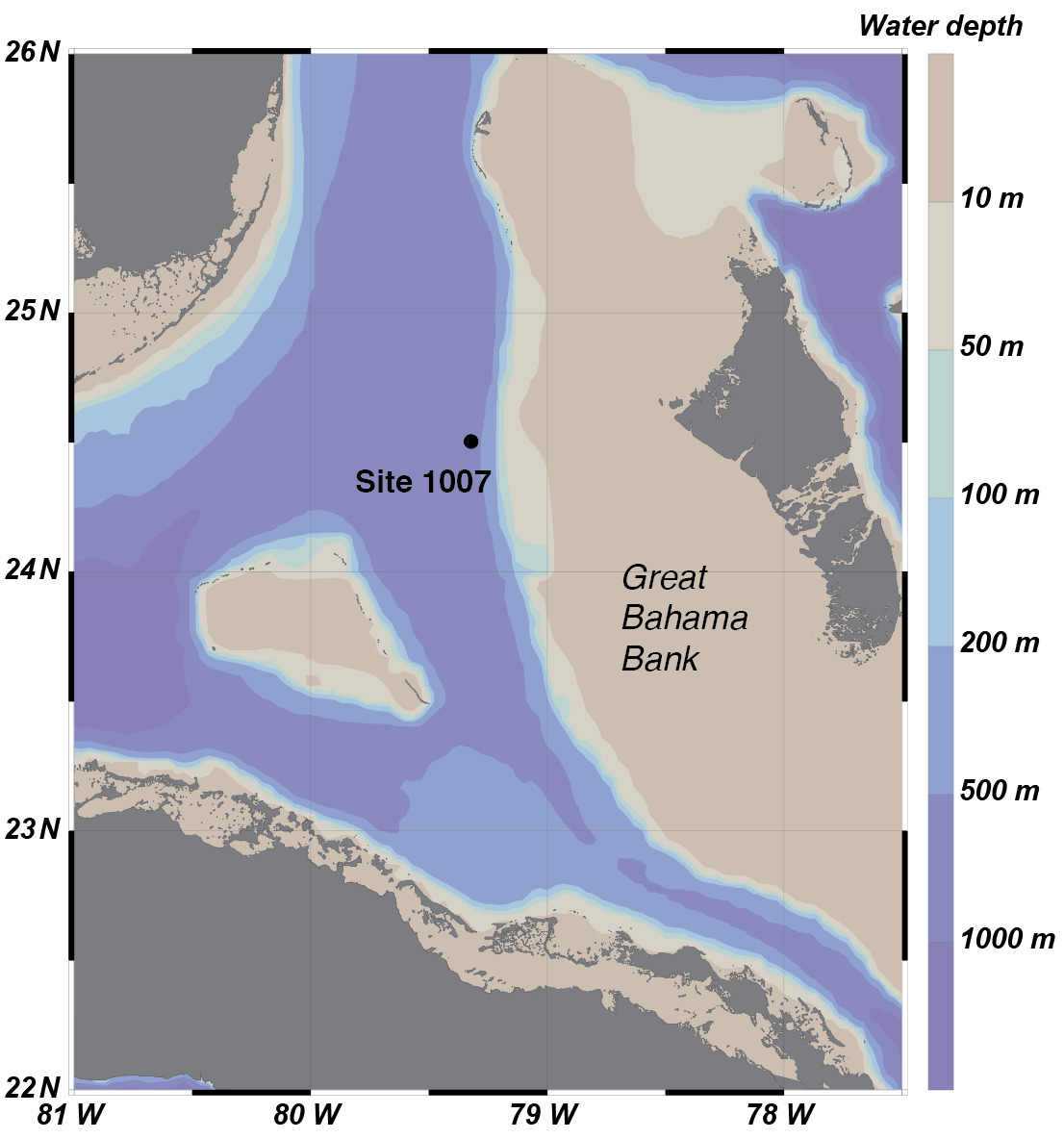
The studied ODP Leg 166 Site 1007 (24°30.261′N, 79°19.34′W, fig. 1) has a total cored depth of 1250 m, which consists mainly of carbonates deposited from the early Miocene to present. The current water depth of this site is around 700 m (fig. 1, Shipboard Scientific Party, 1997). This site is rich in foraminifera fossils, and an age model based on nannofossil zonation shows that it was formed during the last 20 Myrs, with sedimentation rates in the range of 0.001 to 0.021 cm yr-1 (Shipboard Scientific Party, 1997). Due to its much greater water depth than the nearby Clino and Unda cores (Swart & Eberli, 2005), meteoric diagenesis has not impacted this site. This is also supported by the δ18Ocarb values (~0 ‰), which do not show negative shifts typical of meteoric diagenesis (e.g., Frank & Bernet, 2000; Stewart et al., 2015). The Site 1007 core thus provides an opportunity to study the effects of marine burial diagenesis on platformal carbonates. The studied sequence can be divided into two mineralogically distinct intervals (fig. 2A). The upper interval (0–203 meters below seafloor [mbsf]) is mainly made up of aragonite (~65 %) and low-Mg calcite (~25 %), with minor contributions from high-Mg calcite (<5 %) (Shipboard Scientific Party, 1997). The lower interval (203–1100 mbsf) is mainly made up of low-Mg calcite (~80 %), with small amounts of aragonite (0–20 %) and dolomite (0–20 %) (Shipboard Scientific Party, 1997).
Site 1007 as well as nearby sites (such as ODP Site 1006) have been extensively studied to understand the influence of marine burial diagenesis on various isotopic systems such as C isotopes (Swart & Eberli, 2005), Li isotopes (Dellinger et al., 2020), U isotopes (Chen et al., 2018), and Ca and Mg isotopes (Higgins et al., 2018). Thus, these sites provide a unique window to pinpoint the effect of marine burial diagenesis on the B isotopic system in platform carbonate sediments, which can be regarded as an ideal analogue for a wide range of commonly analyzed ancient carbonate rocks.
2.2. Sample digestion
A total of 21 samples from the studied site were analyzed for B/Ca and δ11B values (fig. 2) following the method described in Foster (2008), Zhang et al. (2017) and Henehan et al. (2019). About 10 mg of powdered bulk samples were weighed for digestion. The carbonate digestion follows the procedures described by Zhang et al. (2017) and Henehan et al. (2019). First, the samples were ultrasonicated and rinsed with MQ water five times to remove clays. Further, an oxidizing mixture (1 % H2O2 with 0.1 M NH4OH) was used to oxidize organic matter present in the samples. In this step, the samples and the oxidizing mixture were heated to 80 oC in a water bath for 15 min, with 15 s ultrasonication every 5 min. Thereafter, 250 μL of 0.0005 M HNO3 were added to the samples to remove re-adsorbed contaminants. Following this, excess 0.5 M HNO3 (<500 μL) were added for 5 minutes to dissolve carbonates and leave behind insoluble clays, if present. Finally, the dissolved samples were centrifuged for 2–5 minutes, and the supernatant was transferred to clean Teflon vials. No residue was observed in the centrifuged samples before the supernatant was extracted.
2.3. B/Ca measurement
A split of the supernatant (20 μL) was used for B/Ca measurement via a Thermo Element XR ICP-MS at the Yale Metal Geochemistry Center (YMGC). A B-clean Teflon barrel spray chamber, torch, and cones were used to reduce background B content. Several in-house standards were used to monitor the uncertainty of the measurement. B/Ca and Ca concentrations of the bracketing standards were also adjusted to match those of the samples. The external precision (2σ) is better than 5 %, as revealed through repeated analysis of the in-house standards and sample duplicates.
2.4. Sample purification
The B purification was done using Teflon columns with 20 μL of 63–100 μm boron-specific anionic exchange resin Amberlite IRA 743 following a well-established protocol (Foster, 2008; Henehan et al., 2019; Zhang et al., 2017). Before the column procedures, B-free acetic acid-Na acetate buffer was added to samples to ensure pH>5. The columns were first cleaned using 2.5 mL of 0.5 M HNO3 and then pre-conditioned using 2 mL of MQ water. The buffered samples were loaded slowly into the columns using pipettes to avoid either the suspension of the resin or the formation of small sample drops on the inner walls of the column reservoirs. Further, 10 aliquots of 160 μL of MQ water were added to remove the matrix from the resin and the inner walls of the columns. Boron was then eluted through the addition of 5 aliquots of 120 μL 0.5 M HNO3. The elution tails were further collected using an additional 120 μL 0.5 M HNO3 to check for incomplete B recovery in sample vials. A total procedural blank and a JCp-1 carbonate standard were added to each batch to monitor potential contamination as well the accuracy of the measurement. All newly constructed columns were tested for potential column fractionation before use by running B-purified NIST SRM boric acid standard 951 and ensuring that each measurement fell within 0.00 ± 0.10 ‰.
2.5. δ11B measurement
Boron isotopic compositions were measured on a Thermo Neptune Plus MC-ICP-MS at the Yale Metal Geochemistry Center (YMGC), following the procedure described in Foster (2008), Zhang et al. (2017), and Henehan et al. (2019). A separate B-clean Teflon barrel spray chamber was used to avoid contamination during measurement. Ammonia gas was added to improve B washout. Samples were bracketed by NIST SRM 951 boric acid reference standards. Boron isotopic results were reported in δ-notation (δ11B) relative to NIST SRM 951. The total procedural blanks were in the range of 0 to 59.9 pg of B, which is much smaller than the size of the samples (>10 ng B). Thus, the influence of total procedural blanks on the δ11B results of samples is minor (<0.2 ‰). The δ11B of the JCp-1 standard was 24.26 ± 0.19 ‰ (2σ, n=4), consistent with reported values (Gutjahr et al., 2021; McCulloch et al., 2014; Zhang et al., 2017). This also suggests an external precision of ~0.2 ‰ (2σ) for these measurements. Two more boric acid standards, AE120 and AE121, were measured to monitor the precision and accuracy of sample measurements (Vogl & Rosner, 2012). The measured values for AE120 and AE121 were -20.33 ± 0.17 ‰ (2σ, n=11) and 19.56 ± 0.15 ‰ (2σ, n=11), respectively. These results compare well with values previously reported by Zhang et al. (2017) (-20.27 ± 0.23 ‰ and 19.54 ± 0.15 ‰, respectively) and Stewart et al. (2021) (19.71 ± 0.07 ‰ for AE121).
2.6. Diagenetic modeling
To simulate the changes we observed in the B/Ca and δ11B values of carbonates in the studied ODP Leg 166 Site 1007, we built a simple diagenetic model that includes five components: the amount of aragonite in sediments, [B] and [10B] of porewater, and [B] and [10B] of carbonates (aragonite and diagenetically formed calcite). The only reaction in the model is the neomorphism of aragonite to low-Mg calcite. Dissolution and reprecipitation processes without a net change in the amount of carbonate were used to simulate this neomorphism process. The mass balance equation for aragonite in sediments (A) is (cf. Berner, 1980):
∂A∂t=−∂∂z(ωA)−kA
where is the advection rate of sediments, k is the rate of neomorphism of aragonite to low-Mg calcite, and z is depth (positive downward). The mass balance equations for the other components are (following Berner, 1980; Tarhan et al., 2021; M. Zhao et al., 2023; M. Y. Zhao et al., 2021; M. Y. Zhao, Planavsky, et al., 2020; M. Y. Zhao, Zhang, et al., 2020):
∂Cl∂t=∂∂z(D∂Cl∂z)−∂∂z(υCl)+k1−ϕϕA(Ri−Req)
∂Cs∂t=−∂∂z(ωCs)−kA(Ri−Req)
where Cl represents [10B] or [B] in porewater, represents [10B] or [B] in carbonates, is the coefficient of molecular diffusion, is the advection rate of pore water, is porosity, and Ri is the initial [10B]/[Ca] or [B]/[Ca] ratio in aragonite. Req represents the [10B]/[Ca] or [B]/[Ca] ratio in carbonates that is in equilibrium with porewater. The effect of tortuosity on diffusion is included as where Dm is the intensity of molecular diffusion in seawater. The original geochemical composition of the carbonates (B/Ca and δ11Bcarb) was set to be the same as that observed in the upper sediments at Site 1007 (<160 m). The effect of compaction on the advection rates of carbonates and porewater was included in the calculation, following Berner (1980). The porosity in the model was set as a function of depth by fitting the real data shown in Shipboard Scientific Party (1997). The fitting equation is where and represent porosity at the top of the sediment column and at depth, respectively, and is the porosity attenuation length. Model components and boundary conditions are shown in table 1. Model parameters can be found in table 2. The main equations for each component in the model are shown in table 3. The pH of porewater is fixed at 7.5, following data reported by Shipboard Scientific Party (1997). The Req of [B]/[Ca] in carbonates was set as a function of porewater [B] ([B]p):
Req=KDb∗[B]p
where is the partition coefficient of [B]/[Ca] between low-Mg calcite and porewater. The for [10B]/[Ca] of carbonates was calculated under the assumption that the B of newly formed calcite is mainly from B(OH)4- of the porewater (pH = 7.5; Shipboard Scientific Party, 1997) at the study site (Noireaux et al., 2015):
([(11B][((10B])cal=([(11B][((10B])borate
where is the 11B/10B ratio of newly formed calcite and is the 11B/10B ratio of borate in porewater. Thus, a series of equations is required to calculate the 10[B]/[B] ratio of newly formed calcite in the main equation of carbonate [10B] from [10B] and [B] of porewater (table 3). First, the 11B/10B ratio of porewater can be calculated from [10B] and [B] of porewater ([10B]pw and [B]pw) using:
([(11B][(10B])pw=[B]pw−[(10B]pw[(10B]pw
The fraction of borate in porewater is a function of pH:
fborate=KBKB+[H]
where is the dissociation constant of boric acid. Further, the 11B/10B ratio of borate can be calculated from and using:
([(11B][(10B])borate=([(11B][(10B])pwfborate+(1−fborate)α
where is the B isotopic fractionation between boric acid and borate, which is set as 26 ‰ following the study of Nir et al. (2015). can then be calculated using equations (5) and (8). Finally, 10B/B of newly formed calcite can be calculated from using:
([(10B][B])cal=11+([(11B][(10B])cal
Following findings that the recrystallization rate of deep-sea carbonates decreases exponentially with depth (Fantle & DePaolo, 2007), the rate of neomorphism (k) is also set as an exponential attenuation function of depth. The attenuation of k with depth is consistent with the fact that aragonite is not fully transformed into low-Mg calcite even at depth (>800 m, fig. 2). The rate was set as zero for the upper 140 m, since the relatively high concentration of aragonite in these sediments does not support neomorphism above this depth. Thus, the expression of the rate constant is:
k={0, z<140 mk0e−z−140λk, z>140 m
where and are the rate constant of neomorphism and the attenuation length, respectively. The parameters and were estimated by fitting aragonite percentage as well as B/Ca and δ11B data of carbonates. Note that the above neomorphism model only describes aragonite and the low-Mg calcite formed through neomorphism. It does not include initial low-Mg calcite in the sediments, which at Site 1007 accounts for ~25 % of carbonate sediments in the upper intervals of the core. Thus, the results of the model were calibrated to consider the proportion of initial carbonate sediments comprised of low-Mg calcite through:
(BCa)fcarb=(BCa)mcarb∗fiarag+(BCa)ical∗(1−fiarag)
where is the final model-generated carbonate B/Ca value after calibration of initial calcite, is the carbonate B/Ca value determined from the above neomorphism model, is the initial portion of aragonite in the sediments, and is the B/Ca value of the initial calcite in sediments, which was set to be the same as the B/Ca value of the final carbonates (bulk carbonate sediments) at depth (45×10-6 mol/mol). Similar to equation (11), the final model-generated carbonate δ11B after calibration of initial calcite is calculated using:
δ(11Bfcarb=δ(11Bmcarb∗(BCa)mcarb∗fiarag+δ(11Bical∗(BCa)ical∗(1−fiarag)(BCa)fcarb
where is the carbonate δ11B resulting from the above neomorphism model, and is the δ11B of initial calcite in sediments.
The model was written in R. The thickness of the sediment pile was set as 1000 m, which was divided into 100 layers of uniform (10 m) thickness. The model was solved numerically using the Method of Lines through the Variable Coefficient Ordinary Differential Equations (VODE) solver (Soetaert et al., 2010). The model was run to steady state to calculate the depth distribution of the geochemical compositions of both pore water and carbonates.
3. Results
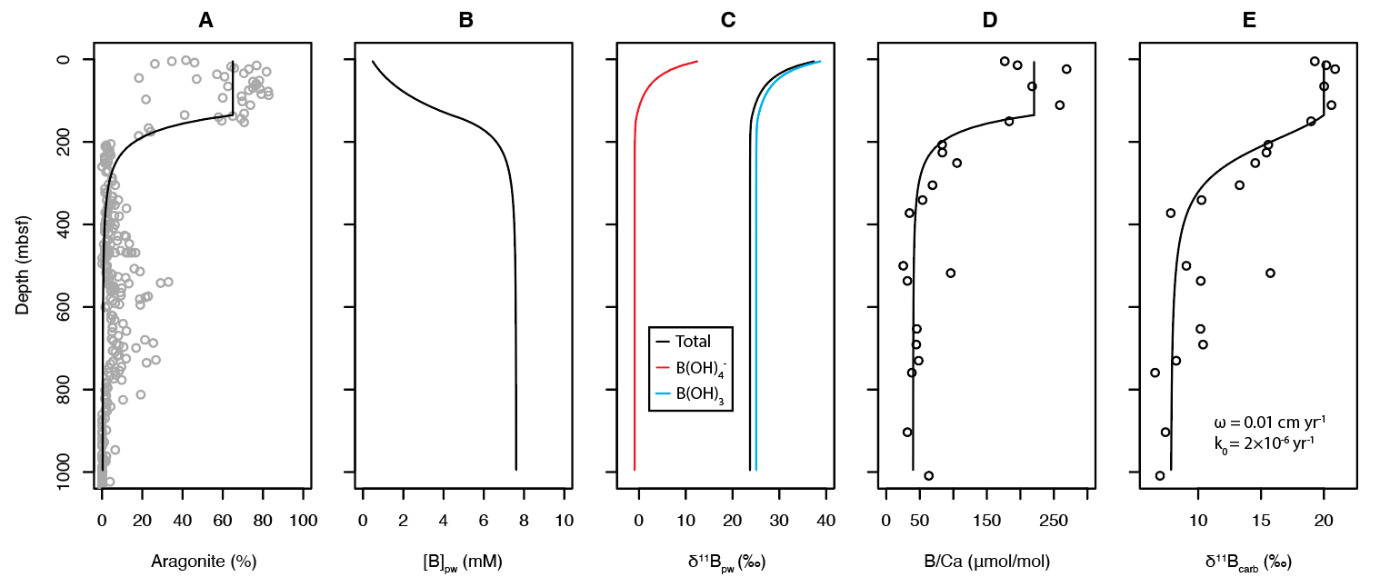
As shown in fig. 2 and table 4, our analyses indicate that both B/Ca and δ11Bcarb values of Site 1007 sediments decrease with depth. B/Ca is approximately 220 μmol/mol for the upper 160 m, followed by a decrease from ~220 to ~50 μmol/mol between 160 and 400 mbsf. Finally, B/Ca stabilizes at ~50 μmol/mol below 400 mbsf. Variation in δ11Bcarb appears to extend deeper than that of B/Ca. The δ11Bcarb value is approximately 20 ‰ in the upper 160 m, with a gradual decrease from 20 ‰ to ~7.0 ‰ between 160 and 750 mbsf. Below 750 mbsf, δ11Bcarb is relatively stable around 7 ‰.
Significant changes in B/Ca and δ11Bcarb occur around 160–250 mbsf. Previous measurements from the core reveal a dramatic change in mineral composition at the same depth (fig. 2; Shipboard Scientific Party, 1997). The percentage of aragonite decreases from ~70 % to ~10 %, whereas the percentage of calcite increases from ~20 % to 80 % (fig. 2). The amount of dolomite also increases below this level, which corresponds closely with a decrease in porewater Mg/Ca (fig. 2; Shipboard Scientific Party, 1997). Moreover, across the same depth range, there is also a considerable decrease in the δ13C value of carbonate from ~4 ‰ to ~ 2 ‰ (fig. 2; Higgins et al., 2018; Swart & Eberli, 2005).
4. Discussion and Diagenetic Modeling
4.1. Evaluation of clay contamination on δ11Bcarb
Clay contamination may alter the δ11B values of bulk carbonates, but we consider the effect to be negligible in our samples, as discussed below. The effect of clay contamination on δ11Bcarb has been explored experimentally, using mixtures of clays and carbonates (Deyhle & Kopf, 2004). The results show that, in a worst-case scenario, six hours of digestion using 2M HCl induced only a 0.14 ppm increase in B content and a 3 ‰ shift in δ11Bcarb in carbonate-dominated mixtures containing 20 % clays. We digested the samples using 0.5 M HNO3 for five minutes. The B contents of our carbonate samples are higher than 3 ppm (table 5), but the clay contents of our samples are lower than 10 %. Thus, the contamination from structurally-bound B in clays during our digestion procedures should be minor. Moreover, B concentration decreases rather than increases with our measured Al content (table 5), which also cannot be explained by clay contamination.
We have additionally evaluated the contribution of desorbable B (Bdesorb) from clays in our measurements (table 5). A typical Bdesorb/Al of 1/9000 for clay was used in this calculation (Paris et al., 2010). The results show that the contribution of Bdesorb to our δ11Bcarb measurements is <1 %. This can generate a maximum δ11Bcarb shift of only -0.08 ‰, even if the assumed δ11B of desorbable B is extremely low (-10 ‰).
4.2. The influence of marine burial diagenesis on δ11Bcarb
The δ11Bcarb values of the top 160 m (~20 ‰) are nearly the same as the pre-industrial δ11B value of seawater borate (δ11Bborate = 19.7 ‰, Zhang et al., 2017), and they are also comparable to the δ11B value of core-top ooids from the Bahamas (~22 ‰, Hemming & Hanson, 1992; Zhang et al., 2017). This suggests no significant B isotopic fractionation between aragonite and seawater borate, consistent with previous experimental findings for synthetic aragonite (Henehan et al., 2022; Klochko et al., 2009; Noireaux et al., 2015; Sen et al., 1994).
Unlike the upper 160 m of the core, the decrease in δ11Bcarb with depth at Site 1007 is difficult to explain as a primary seawater or depositional signal. Given that reconstructed seawater δ11B is never more than ~2 ‰ lighter than the modern seawater value (~38–40 ‰) across the 20-Myr depositional duration of the core (fig. 3; cf. Greenop et al., 2017; Rasbury & Hemming, 2017), and estimated changes in atmospheric pCO2 and marine pH (Beerling & Royer, 2011; Rae et al., 2021) are not large enough to have been the primary drivers of the ~13 ‰ shift we observe, changes in seawater chemistry alone cannot fully explain this decrease in δ11Bcarb.
An alternative explanation for the observed decrease in δ11Bcarb with depth is a change in primary mineral composition and thus B isotopic fractionation between seawater borate and precipitated carbonates. The major mineralogical shift starting from around 160 mbsf at Site 1007 has been explained as reflecting a sediment source change from pelagic low-Mg calcite (i.e., coccoliths and foraminifera) to peri-platform aragonite (derived from calcifying green algae and non-skeletal carbonates) (Swart, 2008; Swart & Eberli, 2005). If a change in mineral composition were the only reason for the observed δ11Bcarb shift, it would imply a significant negative fractionation between low-Mg calcite and seawater borate (∆carb-borate = ~-11 ‰). This is, however, much larger than the known fractionation factors between calcite and borate, which are less than 4 ‰ (Henehan et al., 2016; Rae et al., 2011; Rasbury & Hemming, 2017). Thus, a change in primary mineral composition is an unlikely explanation for the observed significant δ11Bcarb decrease with depth. Lending some support to our conclusions, a previous study on Ca isotopes at Site 1007 found that changes in primary mineralogy were unlikely to be a driver of Ca isotopic variability (Higgins et al., 2018).
Given our results, a more plausible explanation is that the original mineralogy of the entire core was aragonite and high-Mg calcite, and that marine burial diagenesis resulted in closed-system neomorphism of aragonite to low-Mg calcite at depth. Under this scenario, B release from aragonite to porewater during diagenesis would explain the decrease in carbonate B/Ca values with depth in the core. Given that most of this B originated from isotopically light seawater borate, porewater δ11B should in this scenario also decrease. Low-Mg calcite precipitating from ambient porewater would then inherit a lighter δ11B value than the original aragonite.
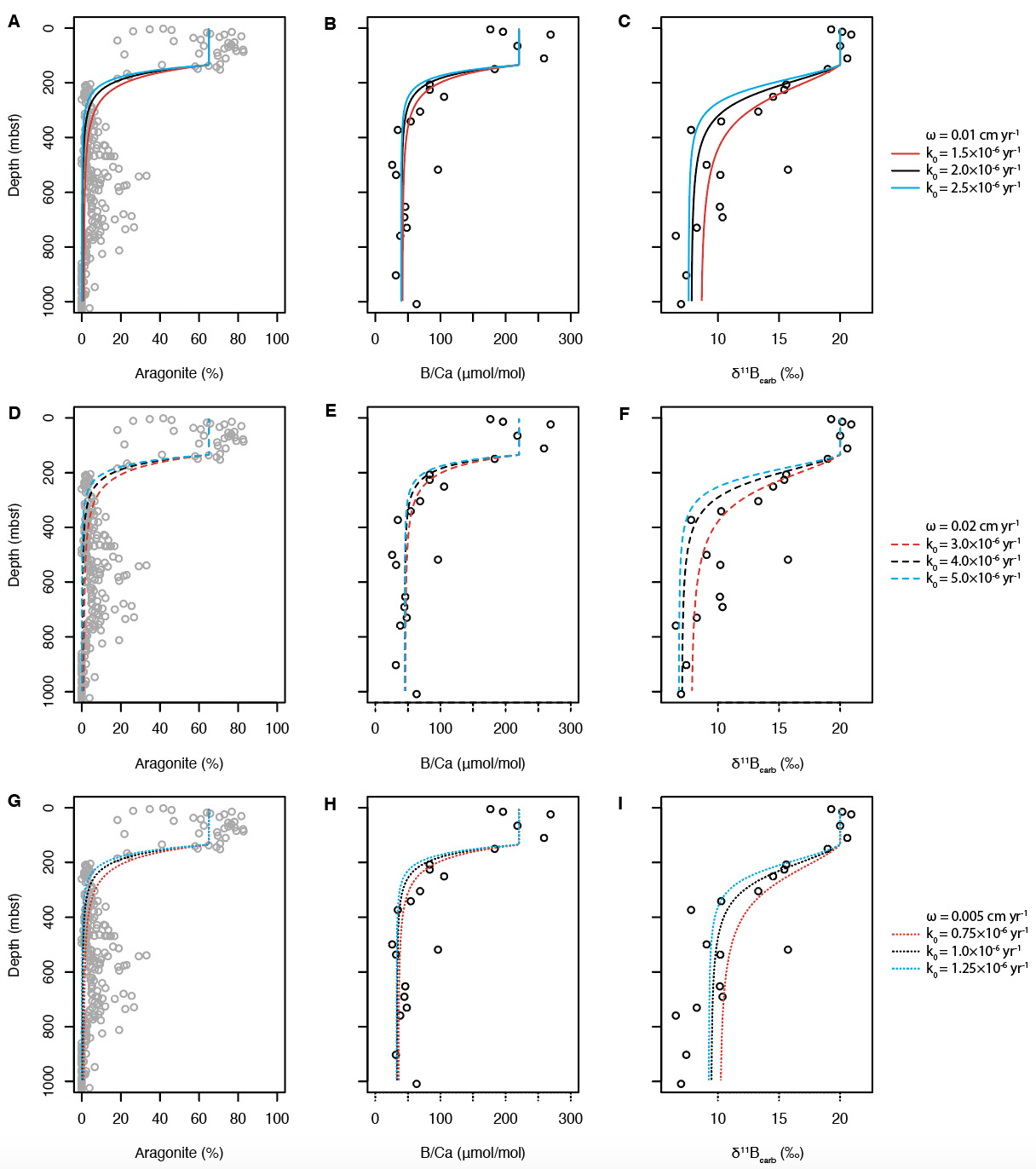
This interpretation is also supported by our diagenetic model (fig. 4). In this simulation (fig. 4), boundary conditions such as the initial B/Ca and δ11B values of carbonates were estimated based on the empirical data reported by Zhang et al. (2017) and this study. Most of the other parameters were obtained from the literature (table 2). A range of values for the rate constant of neomorphism and attenuation length for the rate of neomorphism are explored in the model simulations, as they have not been previously constrained. The average sedimentation rate of the study site is ~0.01 cm yr-1, based on the age model developed from nannofossil and foraminiferal biostratigraphic constraints (Shipboard Scientific Party, 1997). However, the sedimentation rates previously calculated for the upper 300 m range from 0.005 to 0.02 cm yr-1 (Shipboard Scientific Party, 1997). As neomorphism occurs chiefly within this same depth range (fig. 2), this variability in sedimentation rate could potentially strongly influence our estimates of neomorphism rate (fig. 5). On the other hand, model runs indicate that changes in do not strongly influence estimates of neomorphism rate. As shown in figs. 4 and 5, with paired and values, the model can reproduce mineral composition profiles as well as empirical carbonate B/Ca and δ11B values. Notably, the model results also replicate our empirical observation that the shift in δ11Bcarb values extends deeper than that of B/Ca values—bolstering our interpretation that downcore changes in boron geochemistry are driven in large part by neomorphism (fig. 4). Similar decoupling between different components has also been observed for a recently developed 2D model for meteoric diagenesis, likely reflecting a mixing effect between fluid endmembers (M. Y. Zhao, Planavsky, et al., 2020). This suggests that carbonate B/Ca and δ11B values are nearly in equilibrium with those of ambient porewater. The results displayed in fig. 4 also indicate that the release of boron from carbonates during neomorphism will induce a significant increase in the boron concentration of porewater as well as a decrease in the porewater δ11B values of total boron, boric acid B(OH)3 and borate ion B(OH)4-. The δ11Bcarb value decreases following neomorphism, due to the preferential uptake of B(OH)4- from pore water (fig. 4). The abnormally high B/Ca and δ11Bcarb values at 518 mbsf are due to the higher aragonite content of this sample (table 6; Shipboard Scientific Party, 1997).
As shown in fig. 5, the value of that results in the closest match between model and empirical data increases following an increase in sedimentation rate For example, the best estimate for the value of is 2×10-6 yr-1 when = 0.01 cm yr-1, but it increases to ~4×10-6 yr-1 when = 0.02 cm yr-1. Considering the variation in sedimentation rate in the upper 300 m, the best estimate for should, in this light, be in the range of 1×10-6 to 5×10-6 yr-1. This compares well with the recrystallization and/or neomorphism rate of shallow water carbonates during subaerial diagenesis, which has been estimated to be in the range of 10-6 to 10-4 yr-1 (James & Choquette, 1984; Matthews, 1968; Steinen & Matthews, 1973; M. Y. Zhao, Planavsky, et al., 2020). These values are higher than the recrystallization rates of deep-sea carbonates, which could be as low as 2×10-8 to 4×10-9 yr-1 (Fantle, 2015; Fantle & DePaolo, 2007). The difference in the reaction rate between carbonate platform and the deep-sea carbonates is likely due to differences in initial carbonate compositions. Modern deep-sea carbonates consist mainly of low-Mg calcite, whereas shallow-water carbonates in platform settings such as the Great Bahama Bank consist largely of high-Mg calcite and aragonite (e.g., Swart & Eberli, 2005). As high-Mg calcite and aragonite are metastable, it is unsurprising that these mineral transformations occur at a higher rate.
Neomorphism occurs well below the sediment-seawater interface (~200 mbsf and deeper) in the study site, suggesting that some triggering factor for the transformation of carbonate minerals must operate at that level, potentially a change in porewater Mg/Ca values (fig. 2; Shipboard Scientific Party, 1997). The decrease in porewater Mg/Ca below 160 m could be driven by the formation of dolomite, which is supported by an increase in the amount of dolomite below this level (fig. 2A). The molar ratio of Mg to Ca has been widely regarded as the most important determining factor for calcium carbonate polymorphism (Balthasar & Cusack, 2015; Hardie, 1996; Stanley & Hardie, 1998). At the seawater temperature measured at Site 1007 (~10 oC; Shipboard Scientific Party, 1997), the laboratory-derived Mg/Ca molar ratio for a dramatic shift from aragonite to calcite is in the range of 3–4 (Balthasar & Cusack, 2015). This compares well with the observed shift of porewater Mg/Ca with depth from 5–6 to <4 across 160 m (fig. 2). These observations further support our interpretation that the change in the distribution of carbonate mineral types across 160 m is driven by the neomorphism of aragonite to calcite rather than a change in the primary (initial) mineral composition of carbonate factory sediments delivered to this site.
Previous work has indicated that there is also lateral fluid flow at Site 1007, as the porewater [Cl-] concentration increases from a seawater value at 20–30 mbsf to substantially higher values below 100–200 mbsf (Higgins et al., 2018; Shipboard Scientific Party, 1997). This lateral fluid (e.g., a brine) may in turn result in fluid-buffered conditions, responsible for the elevated δ44Cacarb values observed by previous studies below ~200 mbsf (Higgins et al., 2018). However, since significant B release to pore water is indicated by the down-core decrease we observe in B/Ca values, δ11Bcarb values may not be as fluid-buffered as δ44Cacarb values. Future study of the source, composition and flow rate of this lateral fluid migration, as well as further characterization of the evolution of porewater B signatures with depth will, we hope, help to further constrain the influence of these factors and refine our predictive framework for diagenetic behavior of B/Ca and δ11Bcarb.
4.3. Implication for the interpretation of ancient δ11Bcarb records
Our results have implications for interpreting the B isotopic composition of ancient carbonates. Previous studies have interpreted the δ11B signatures of ancient bulk carbonates (i.e., the composite signature of all carbonates in a sample) as a reflection of seawater pH and thus atmospheric pCO2 (e.g., Clarkson et al., 2015; Kasemann et al., 2005, 2010; Ohnemueller et al., 2014). Our results, however, indicate that bulk carbonates can experience a significant δ11B decrease (as much as 12 ‰—i.e., a change that, in the traditional B isotope framework, might be interpreted as a shift in seawater pH from 8.2 to <7) during marine burial diagenesis. This suggests that even so-called “well-preserved” carbonates that have experienced only marine burial diagenesis may be unable to faithfully preserve seawater-derived δ11Bcarb signals. We acknowledge that the degree of alteration in δ11B is likely to be strongly site-specific (Edgar et al., 2015; Spivack et al., 1993; Spivack & You, 1997), depending on the original mineral composition as well as porewater conditions. While recrystallization and decreases in δ11B values of bulk carbonates have been observed in a deep-sea site dominated by foraminifera and nanofossils (ODP Site 851; Spivack & You, 1997), other studies have not observed similar degrees of B isotopic resetting in other deep-sea sites consisting mainly of foraminiferal calcite (Edgar et al., 2015; Spivack et al., 1993). It is worth noting that the primary low-Mg calcite (~25 % of carbonate sediments in the upper portion of the core; fig. 2) at Site 1007 may not have undergone recrystallization. Thus, in situ (and petrographically constrained) measurement of δ11Bcarb in primary low-Mg calcites may provide a better record of seawater signals.
Previous studies have found that the B/Ca and δ11B values of ancient bulk carbonates on continental margins (5 to 30 μmol/mol and -6 to 14 ‰; Clarkson et al., 2015; Kasemann et al., 2005, 2010; Ohnemueller et al., 2014; Vengosh et al., 1991) are much lower than that of modern carbonates deposited in analogous settings (~200 μmol/mol and 20 ‰). This disparity has been variably interpreted to reflect the influence of terrestrially sourced boron (Vengosh et al., 1991), recrystallization (Ishikawa & Nakamura, 1993) or meteoric diagenesis (Stewart et al., 2015). Our results indicate that, in addition to changes in seawater δ11B through Earth’s history (e.g., Rasbury & Hemming, 2017), neomorphism during marine burial diagenesis could also have contributed to the low B/Ca and δ11B of ancient bulk carbonates formed on continental margins.
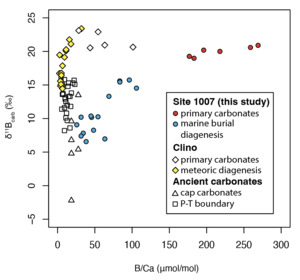
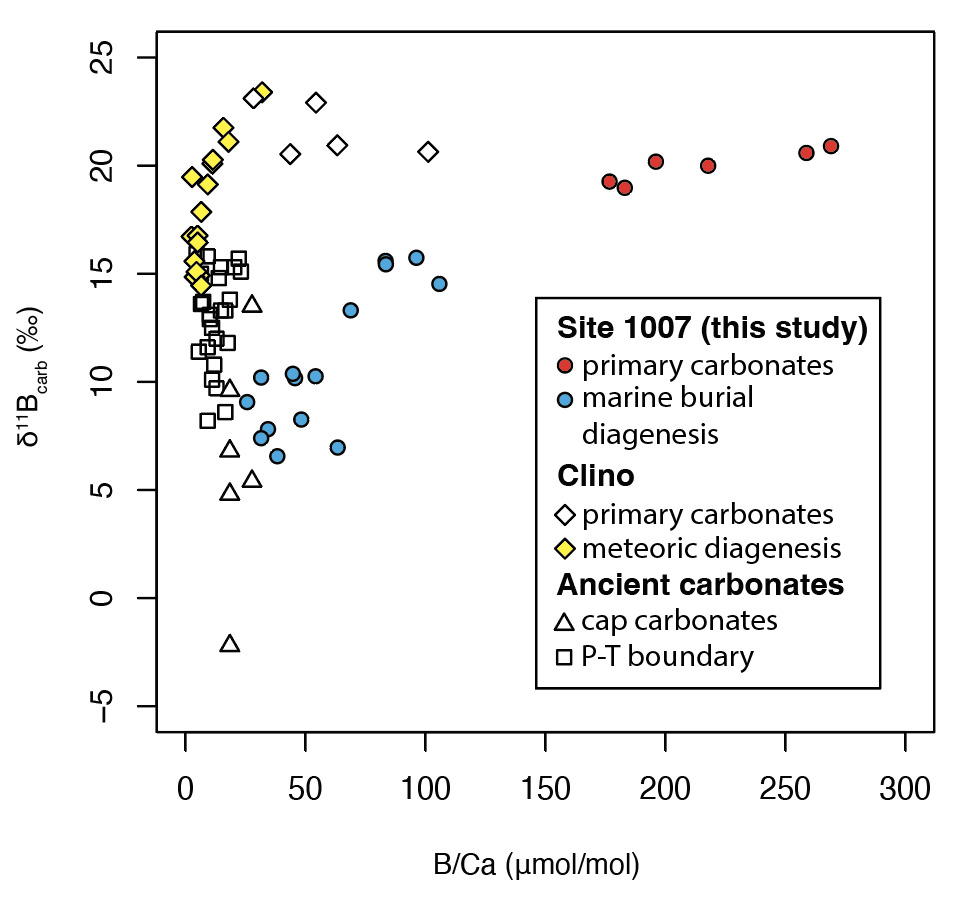
As shown in the cross-plot of B/Ca and δ11B values of bulk carbonates (fig. 6), carbonates subjected to marine burial diagenesis from ODP Site 1007 show higher B/Ca but even lower δ11B values than those subjected to meteoric diagenesis in the adjacent but more proximal Clino core (Stewart et al., 2015). This suggests that meteoric diagenesis may not necessarily be associated with lower δ11B values than marine burial diagenesis, especially when the initial mineral composition is primarily aragonite. Considering the multiple factors shaping both the B/Ca and δ11B values of carbonates, patterns in B/Ca and δ11B space (fig. 6) should be considered in the context of independent geochemical evidence—such as mineral composition and carbon and oxygen isotope values—to distinguish between meteoric and marine burial diagenesis. For instance, the δ18Ocarb values of the studied ODP Site 1007 are close to 0 ‰ and do not show an obvious negative shift in the whole profile, indicating marine burial diagenesis with limited influence from meteoric water.
4.4. Implication for B isotopes as a proxy for carbonate recrystallization/neomorphism
Our results along with those of Stewart et al. (2015) suggest that recrystallization/neomorphism during both meteoric diagenesis and marine burial diagenesis will have a significant impact on carbonate B isotope signatures. This implies that B isotopes could be a proxy for carbonate recrystallization/neomorphism and original carbonate mineralogy. Since there is a significant drop in B isotopic values during recrystallization/neomorphism from high-Mg calcite and aragonite to low-Mg calcite, B isotopes thus may help to identify recrystallization/neomorphism in ancient carbonates in instances for which ambient seawater δ11B values can be robustly estimated. The original seawater B isotopic values can be estimated through analysis of known low-Mg calcites (mainly shells).
5. Conclusions
Geochemical measurements of B/Ca and δ11B values in modern platform carbonates deposited below the zone of typical meteoric diagenesis (~700 m water depth) indicate that substantial decreases in both B/Ca and δ11B values can occur during marine burial diagenesis. Multiple lines of evidence, including the B/Ca and δ11B values of carbonates and porewater Mg/Ca values indicate that notable increases in neomorphism from aragonite to calcite occur at around 200 m below the seafloor. Decreases in δ11B values with depth are likely due to the release of isotopically light B (δ11B ~20 ‰) from carbonate to porewater during neomorphism, with subsequent uptake of the isotopically lighter borate ion (δ11B ~-1 ‰) by the newly formed low-Mg calcite. These interpretations of our empirical data are also supported by numerical diagenetic modeling. Further, our diagenetic model suggests a neomorphism rate in the range of 1×10-6 to 5×10-6 yr-1, which is similar to the recrystallization and/or neomorphism rates proposed by previous studies for meteoric diagenesis. This significant observed change in δ11B values during marine burial diagenesis indicates that the δ11B signature of bulk carbonate sediments does not, in contrast to recent assertions, necessarily provide a simple proxy for the pH of ancient seawater. Our results also imply that B isotopes could be employed as a proxy for carbonate recrystallization/neomorphism and original carbonate mineralogy.
Acknowledgments
We thank Shuang Zhang, Donald Penman and Dan Asael for their assistance with laboratory analyses. M.Z. is funded by the IGGCAS Key programme (no. IGGCAS-202201) and the programme of the Chinese Academy of Sciences (E251520401). NJP acknowledges support from the Packard Foundation. We are grateful to Joe Stewart, Sean Murray and Jesse Farmer for insightful reviews that improved this manuscript.
Data Availability
https://doi.org/10.17632/bxh8gmxp9f.1, https://data.mendeley.com/datasets/bxh8gmxp9f/1
Editor: C. Page Chamberlain, Associate Editor: Kimberly Lau