1. Introduction
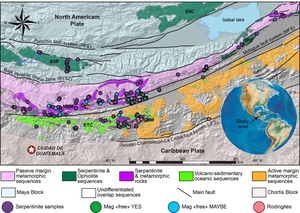
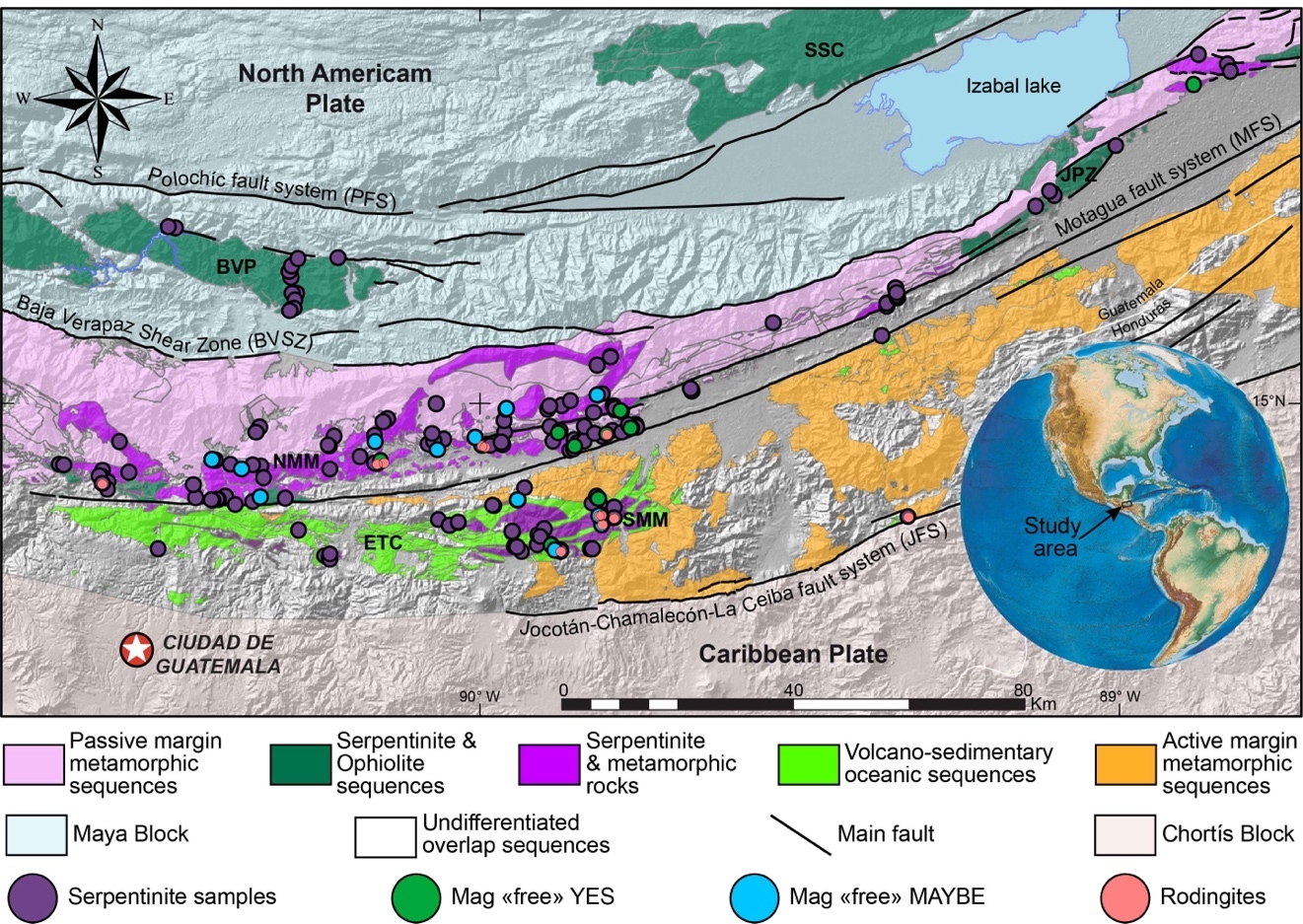
The Motagua Fault System (MFS) of Guatemala is interpreted as the active plate boundary between the North American and Caribbean plates with accumulated displacement of ~103 km. Belts of serpentinite are exposed on both sides of the MFS are critical to the expression and interpretation of the MFS. They are present throughout most of its exposure in central Guatemala, over 350 km from the Caribbean Sea to the western extent near the border with Mexico where they are buried in young volcanics. They have been interpreted as mélanges containing tectonic inclusions of high-pressure, low-temperature (HP–LT) rocks, including jadeitite, eclogite and various metabasites as well as low-pressure, low-temperature (LP–LT) assemblages more characteristic of an ophiolite (e.g., Brueckner et al., 2009; Harlow et al., 2003, 2004). Obducted ophiolites, with aspects of ocean-crust layering, are exposed mostly to the north in the Baja Verapaz and Sierra de Santa Cruz ultramafic complexes. The MFS-related serpentinite belts contain HP–LT lithologies that represent emplacement via exhumation of subduction channel assemblages as blocks, so-called “knockers.” Consequently, the zone has earned the additional name—the Guatemala Suture Zone (GSZ; Flores et al., 2013).
The juxtaposition of mélanges of different age and origin invites an examination of the context of the ultramafic hosts. Much work has been done to interpret the characteristics of serpentinites in different tectonic settings but often with assumptions about the control of the setting upon the nature of the ultramafic protolith rather than attempt to separate the protolith from the hydration and its compositional imprint.
The juxtaposition of mélanges of different ages across a strike-strike slip fault is odd, and a robust interpretation has not been determined. Thus, an extensive examination of the serpentinites and comparison are needed to assist interpretation of similar occurrences. Understanding the origin and processing in these serpentinites is critical to interpreting the greater aspects and some complexities of the MFS. This work represents an effort to enhance understanding of these serpentinites and the record they contain.
2. Geological Context
The North American-Caribbean plate boundary passes through Central Guatemala along a transform fault system, the MFS. An expanded view of the geology here has been called the Guatemala Suture Zone (GSZ), which is a fault-bound region in central Guatemala that contains the Motagua Fault System (Flores et al., 2013). The GSZ is bounded north and south by two continental crust blocks (Maya and Chortís, respectively) and contains various high-grade schist and gneisses, accreted metavolcanic-metasedimentary sequences, low-grade schists and metasediments, serpentinite mélanges and obducted ophiolites. The serpentinite belts occur north and south of the MFS, and two distinct serpentinite-matrix mélanges separated by the MFS enclose high-pressure–low-temperature (HP–LT) rocks such as eclogite, jadeitite, garnet-amphibolite, and blueschist (Brueckner et al., 2009; Harlow et al., 2004; Tsujimori et al., 2006a). Based on the association of a serpentinite matrix, HP–LT blocks, and vein-related metamorphic rocks, these mélanges have been interpreted as examples of subduction channel (deep mantle wedge) sequences exhumed from depths up to 80-100 km (Flores et al., 2013; Martin et al., 2016, 2020). In addition to the mélanges are fault bound sections of LT/LP serpentinites, often displaying rodingite veins, pervasive brittle fractures and reasonably well-preserved protolith textures, more typical of ophiolitic serpentinite. Large, dismembered ophiolite sequences (Sierra de Santa Cruz—SSC and Baja Verapaz—BVP) are found at the northern extent of the GSZ, thrusting over the Maya block’s passive margin sedimentary sequences (fig. 1). Smaller ophiolitic slivers (e.g., Juan de Paz [JDP] and portions of the El Tambor Complex [ETC]) also occur north and south of the MFS tectonically associated with various metamorphic sequences and serpentinite mélanges (Flores et al., 2013).
The ultramafic belts cutting through central Guatemala were studied by McBirney (1963), and Bertrand and Vuagnat (1976, 1977, 1980). The serpentinites in Baja Verapaz north of Salamá were recognized as part of an ophiolite, and the serpentinites immediately north of the central strand of the MFS (Cabañas fault) were recognized as a mélange but not the full extent. The serpentinites south of the MFS were missed and only described later (Deschamps et al., 2013; Harlow et al., 2004, 2015). On the north side of the Motagua fault is a discontinuous series of mélange units that extend more than 200 km in east-west extent that have been labeled as the North Motagua Mélange (NMM; Flores et al., 2013). This unit also contains jadeitites and eclogites associated with serpentinite mélange that suggest PT conditions varying from 0.6 to 2.1 GPa and 300-620 °C (Flores et al., 2013, 2017). South of the MFS is a much smaller extent of serpentinite-matrix mélange, extending only tens of kilometers along the fault zone and has been labeled the South Motagua Mélange (SMM; Flores et al., 2013). This unit also contains jadeitite and eclogite (principally lawsonite eclogite) blocks that record higher pressures of 1.0-2.6 GPa and lower temperatures of 300-400 °C (Flores et al., 2013; Tsujimori et al., 2006a, 2006b). Although this PT estimate has been contested by Endo et al. (2012), a separate study of elemental zoning in eclogite garnet supports the original interpretation (Bradley, 2019). The SMM serpentinites display very similar mineral assemblages as their northern counterparts but generally lack carbonates.
This study surveyed serpentinites primarily from the NMM and SMM but includes some from ophiolite bodies in the region (BVP, JDP, and ETC) for comparison (see in Supplementary information table S1). Because of the far greater distribution of serpentinites in the NMM compared to the SMM, the number of samples collected and then studied is far greater as well (122 versus 32 locations and 62 versus 29 studied rocks).
3. Analytical Methods
3.1. Optical Microscopy
Petrographic thin sections were prepared from most samples to assess microfabric and mineralogy. These were observed in plane-polarized and cross-polarized light for transparent minerals. Opaques were examined in reflected light to differentiate oxides from sulfides and to detect optical anisotropy. The serpentine minerals can be highly deformed, making it difficult to differentiate antigorite (Atg) from lizardite (Lz) optically, although the former frequently has anomalous blue dispersion in lathy crystals in cross-polarized light. Chrysotile, the rolled or polygonised variety of lizardite, most distinctive when asbestiform, appears to be limited to the ophiolitic samples.
3.2. Magnetite Testing and Image Analysis of Thin Sections
Rather than attempt modes on thin sections or magnetic susceptibility on rock samples, a quick and crude measure of magnetite content was assessed with a REE magnet (~3mm dia.) being held to the exterior of a hand sample. When the magnet would not stick anywhere the rock was considered “not attracted to a magnet”, so called “not magnetic”. If the magnet stuck in spots but not strongly, then it was rated “slightly magnetic”. If the magnet generally stuck to the rock (usually with magnetite showing), the rock was considered “magnetic.”
To obtain a semi-quantitative assessment of magnetite abundance among samples with analytical data and within our capabilities, ImageJ (Open Source) was used to assess the amount (areal) of opaques in thin sections of Atg serpentinites. Thin section images were “bleached” of areas outside the rock, the area of the rock was measured (pixels). Then the opaque (black) areas were determined by thresholding and viewing the results for consistency with the appearance of the thin section. The percentage of opaque in the section is the ratio of “black” area to rock area times 100. We recognize this approach is crude; however, magnetite grains or aggregates as observed by BSE in thin section were not generally submicroscopic so as to be “invisible.” Moreover, the results below are consistent with the compositional inferences.
3.3. X-ray Diffraction
An assessment of the mineralogy of the serpentinites was carried out using X-ray diffraction at AMNH. Many researchers now prefer Raman spectroscopy for microscopic phase identification. Access to Raman for this long-term study has been limited; simply put we have had no access to Raman until very recently. In our recent experience we have found Raman to be an inadequate/unreliable method for distinguishing between antigorite and the other serpentine minerals, compared to XRD and petrography, particularly in instances where both lizardite and antigorite coexist in the same rock. Ongoing research will address this issue. Some measurements were performed on blocks used for making thin sections and some on powders (magnetite magnetically removed after preliminary crushing) with a Philips PW-1710 automated (Bragg-Brentano) powder diffractometer with a diffracted beam monochromator and Cu Kα radiation. Later a Rigaku DMAX/Rapid microdiffraction system with incident monochromatized Cu Kα X-rays, was employed using a Debye scattering geometry into a cylindrical image-plate detector (-30° to 140° 2Θ). Collimators of 0.8 mm (pinhole) to 0.1 mm (monocapillary) were used to irradiate grains or small areas on the surface of the rock blocks. Grains (typically < 0.5 mm across) were glued to a glass fiber attached to a rod and blocks (thick sections or thin-section cutoffs) were attached to a stage. Both were rotated 180 degrees on one axis [phi] and 30–45 degrees on a second axis [omega] at 45 degrees to the phi axis (like a Gandolfi camera motion) to access many diffractions. Most recently a Bruker D8 Advance instrument in Debye Θ-Θ geometry was employed with Cu radiation using a Göbel mirror for monochromatic parallel beam geometry, a 1mm beam-size collimator and a Dectris Eiger2 R 500K area detector on fixed block samples at the Metropolitan Museum of Art. Results yield intensities that depend on sample orientation and the degree of randomness in orientation; however, the values of 2Θ (d) only depend on diffracting minerals. The later approaches permit obtaining a diffraction pattern from small areas or volumes, thus called microdiffraction. Diffraction results were interpreted using JADE (MDI) software and the 2004 PDF-2 diffraction database (ICDD) to “identify” the mineral phases present as well as by comparing with our own numerous diffractograms. The database contains a collection of diffraction data from 5108 minerals plus synthetic patterns of 8362 minerals from the Inorganic Crystal Structure Database (ICSD); matches may be closest to a particular polytype of a serpentine mineral, but we made no attempt to interpret the particular polytype(s) in our samples. Moreover, highly congruent matches of the database standards to measured XRD data are mostly restricted to better crystallized samples. Given the compositional results presented here, and the complexity of real serpentines, this is not surprising.
Chrysotile matches come up commonly for lizardite, whether with Ctl or not. It should be noted that a conspicuous aspect of diffraction from chrysotile is peak broadening with asymmetry that extends to the high-angle side of a peak in a diffractogram and tends to conjoin peaks typical of Lz polytypes 1M and 6T, which would also be the case for deformed or nano-grain size of these lizardites.
3.4. Electron Microprobe/Electron Imaging
To obtain chemical compositions and microtextural information, via back-scattered electron (BSE) images, a Cameca SX100 electron microprobe at AMNH was employed (no BSE images are reported in this paper). Analyses were obtained operating at 15 kV and 10 nA sample current, employing a point beam (~1µm diameter). Natural minerals were used as standards, and the PAP correction scheme according to Pouchou and Pichoir (1991) was employed. For all elements detection limits are < 0.01 wt%. No post processing corrections were made for the effects of structural “water” upon the intensity data from hydrous minerals. Crystalline phases are abbreviated according to Whitney and Evans (2010) unless otherwise specified. Estimates of Fe2+ and Fe3+ were made only for stoichiometric phases like spinels and pyroxenes using an algorithm similar in concept to that of Finger (1972) to calculate Fe3+ from total Fe. Cation charge must sum as close as possible to twice the number of oxygen atoms plus the number of univalent anions (e.g., 6 oxygens in pyroxene), with a maximum permissible sum of cations determined by the crystal’s stoichiometry (e.g., four for pyroxene as above; vacancies in large cation sites are permissible – see Harlow et al., 2006, for more details).
4. Studied Samples
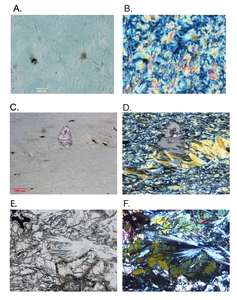
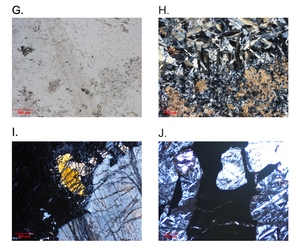
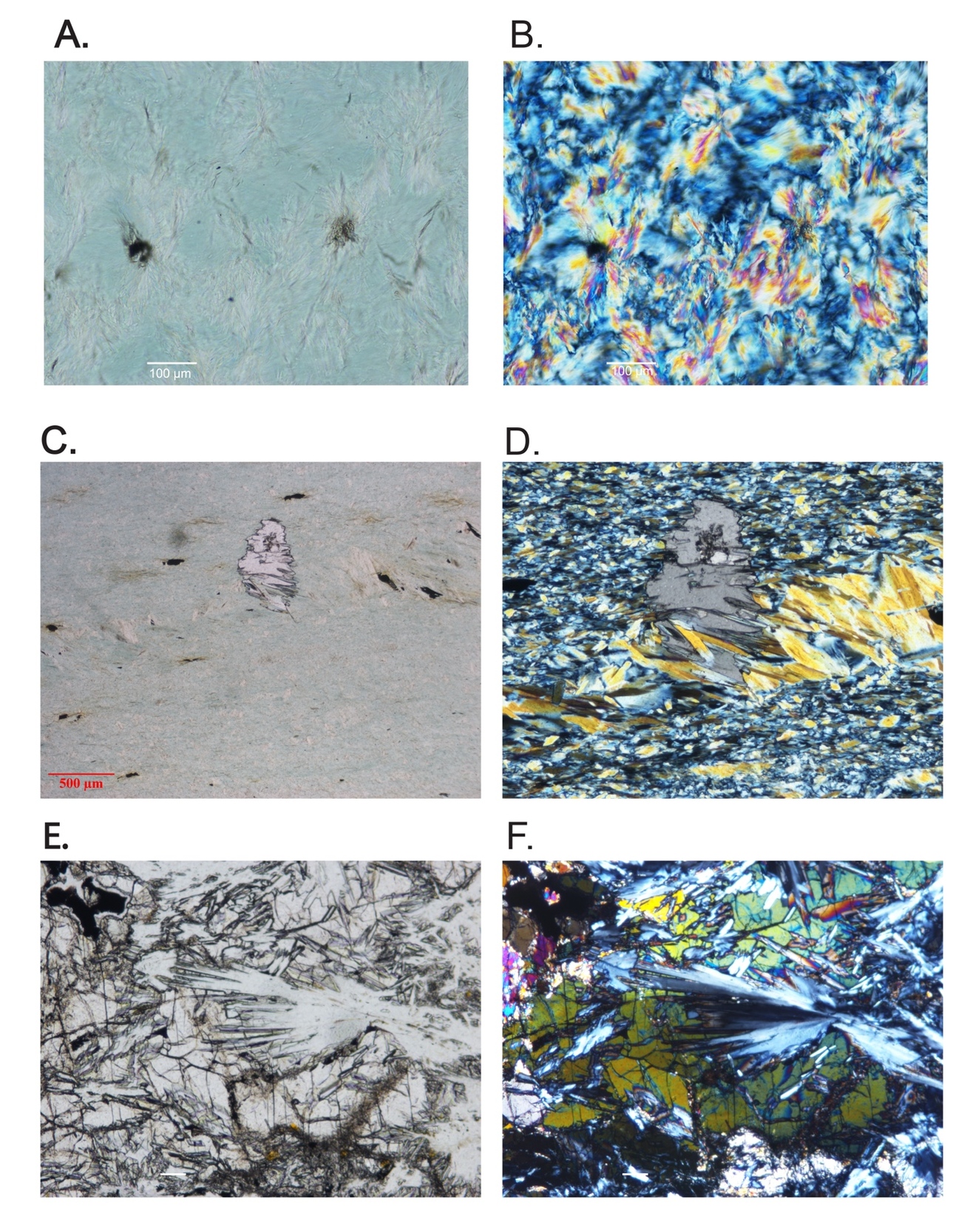
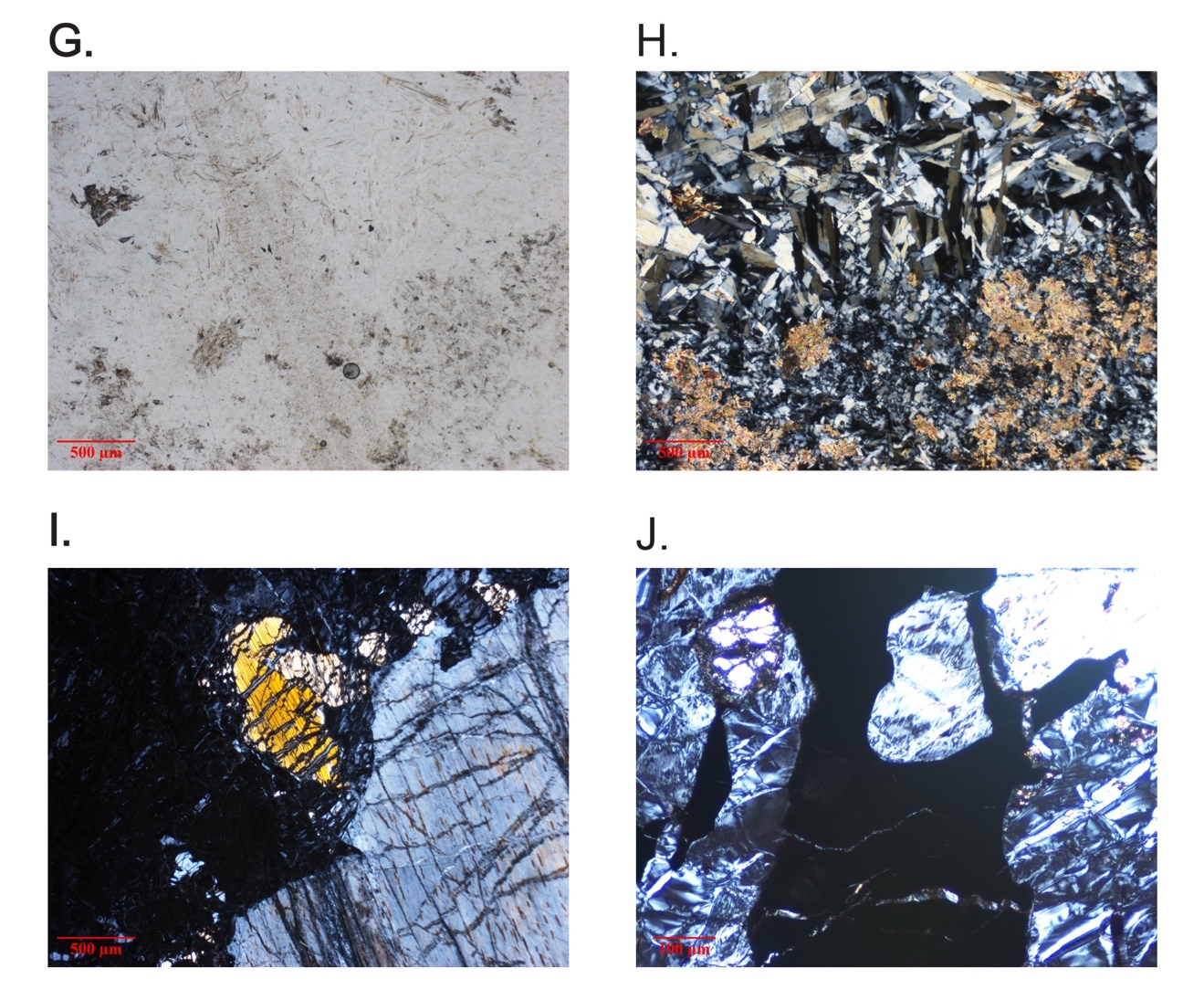
As noted above, the serpentinites of the two mélange zones outcrop in distinct bodies (fig. 1), bounded by faults to the north and south, and consist primarily of antigorite or, uncommonly, Lz-Ctl. Serpentinites can be enigmatic rocks in highly faulted terrains because macroscopic textures can vary across an outcrop from being tough and coherent to highly fractured and almost fissile. Geologists from Guatemármol (the company that exploits serpentinite in the region) learned that larger ellipsoidal blocks, up to kilometers in dimension, were sufficiently competent to be suitable for quarrying for dimension stone, whereas the encapsulating sheared matrix of serpentinite and mélange inclusions was unsuitable. Our sampling includes both types. The large blocks consist just of antigorite as the serpentine mineral, and these are usually completely serpentinized. Figure 2 shows petrographic micrographs of textures in thin sections. Common textures consist of lathy to bowtie-shaped Atg crystal clusters (described as lepidoblastic texture by Evans et al., 2012 and as interpenetrating texture by Wicks & O’Hanley, 1988; see fig. 2A-B here) or heavily sheared microtextures (MVE03-20-4; fig 2C-D); both preclude a petrographic interpretation of the protolith. A small number of samples contain relict pre-serpentinization phases, most typically chromite and clinopyroxene exsolution lamellae in serpentinized orthopyroxene (bastite). A very few specimens from the mélanges are essentially unaltered peridotites with Atg restricted to grain boundaries and fractures. In these, Atg laths “spear” olivine grains with the olivine adjacent to the lath increasing in Fa content and exhibiting local equilibration (see section 5.4 and figs. 2E-F). Antigoritites from the NMM commonly contain phenoblasts (the metamorphic equivalent of igneous phenocrysts, or alternately termed neoblasts) of magnesite, dolomite, or ankerite (e.g., MVE12-74-1; figs. 2G-H), particularly in late-stage veins. Other secondary minerals include magnetite, ilmenite, talc, clinochlore, and sulfides. Andradite was found only in rocks from the SMM and BVP. Mixed serpentine minerals were found in brittlely deformed serpentinites (see discussion). Lz-Ctl serpentinites can be conspicuous because of glassy fracture surfaces in outcrop, lack of large-scale coherence, and, on occasion, the presence of rodingite veins. They are also more likely to manifest relict textures, particularly bastite and hour-glass serpentine textures, typical of replacement of former olivine (Wicks & Whittaker, 1977), together indicating a harzburgite protolith, as well as cross-vein Ctl in fractures. Chromite is commonly preserved, surrounded by ferritchromite (Kimball, 1990; Mellini et al., 2005) or magnetite. Magnetite is common, but carbonates and talc are rare.
The ophiolite bodies only contain Lz-Ctl serpentinite and are virtually identical to the Lz-Ctl rocks from the mélanges, with bastite and hour-glass textures (MVJ84-20-4; figs. 2I–J), comparable mineral constituents, and irregularly-spaced rodingite veins. The El Tambor formation (a more metabasalt-dominated unit adjacent to the SMM south of the Río Motagua) contains selvages of serpentinite comparable to that from the ophiolites.
Some serpentinite bodies are cut by rodingites, which are interpreted as metasomatic replacements of gabbroic veins or bodies by calcic minerals [diopside, (hydro)grossular, vesuvianite, etc.] in oceanic peridotite during serpentinization (Bach & Klein, 2009; Coleman, 1967). The rocks are very fine-grained with a gray or tan color verging on pink. Samples are listed at the end of table S1. Most are associated with Lz serpentinites, but one occurrence, in particular, is associated with both Atg and Lz as well as an odd variety of jadeitite containing both grossular and pumpellyite-Mg (see Harlow et al., 2011).
5. Detailed Mineralogy
5.1. Serpentine minerals
XRD was used to determine mineralogy, and most samples exhibit either Atg or Lz (or Lz-Ctl) but not rarely both, as shown in figure 3. These few samples clearly have mixed Atg and Lz (see table S1) as evident from broad asymmetrical peaks, particularly in the region of 18-22° and 33-37° 2Θ, see figure 4. Also in figure 4, MVE04-18-3 is conspicuous for having Atg matrix (lighter colored in thin section) surrounding (darker colored clasts) Lz, and is cut by veins filled with cross-vein fibrous Atg that represents the last mineralization event. Atg appears to have replaced much of the Lz.
Microprobe analyses of serpentine cannot detect H (or H2O), so compositional comparisons must be based on anhydrous stoichiometry. Ideally, Lz is Mg3Si2O5(OH)4 which can be rewritten as Mg3Si2O7 – 2(H2O). The undulating layer structure of Atg modifies the simple stoichiometry of Lz; the periodicity of the undulations is variable, but the most common spacing is about 17 tetrahedral repeats (represented as m; Capitani & Mellini, 2004; Mellini et al., 1987; Zussman, 1954), which results in the loss of 3 brucite units [Mg(OH)2] from the idealized formula, so
17 Mg3Si2O5(OH)4−3 Mg(OH)2=Mg48 Si34O85(OH)62=Mg48Si34O116+31 H2O
For comparison with the ideal (lizardite) formula, we normalize the above stoichiometry to seven oxygens and separate the water molecules, which yields Mg2.897Si2.052O7 + 1.871(H2O). This enables comparing microprobe analyses of the serpentines by normalizing all analyses to a seven-oxygen basis. Some antigorite analyses have slightly higher Si (or T) content in antigorite. Review of the literature on the minimum reasonable tetrahedral repeat in the structure yields m = 13 (Wunder et al., 2001, and citations above), which should still result in three lost brucite units, yielding the formula Mg2.864Si2.068O7 + 1.830(H2O). The significance will be evident in figures 5 and 6.
The major elements in serpentine are the divalent cations Mg, Fe, Mn, Ni, and Co, the trivalent cations Al and Cr, and Si. Ferric iron is found in serpentine minerals, particularly Lz (see Debret et al., 2014; Eberhard, Plümper, et al., 2023; Evans, 2008; Evans et al., 2012) but cannot be differentiated from ferrous iron in microprobe analyses. Thus, all Fe has been evaluated as ferrous iron – Fe2+in serpentine minerals. A discussion of using serpentine crystal chemistry to estimate ferric iron follows in the discussion section 6.2. Representative microprobe analyses are presented in table 1.
The Tschermak’s exchange is used for accommodating aluminum in the crystal structure:
(4Al+(6Al⇔Si+(Mg+Fe+2+Mn+Ni+Co)
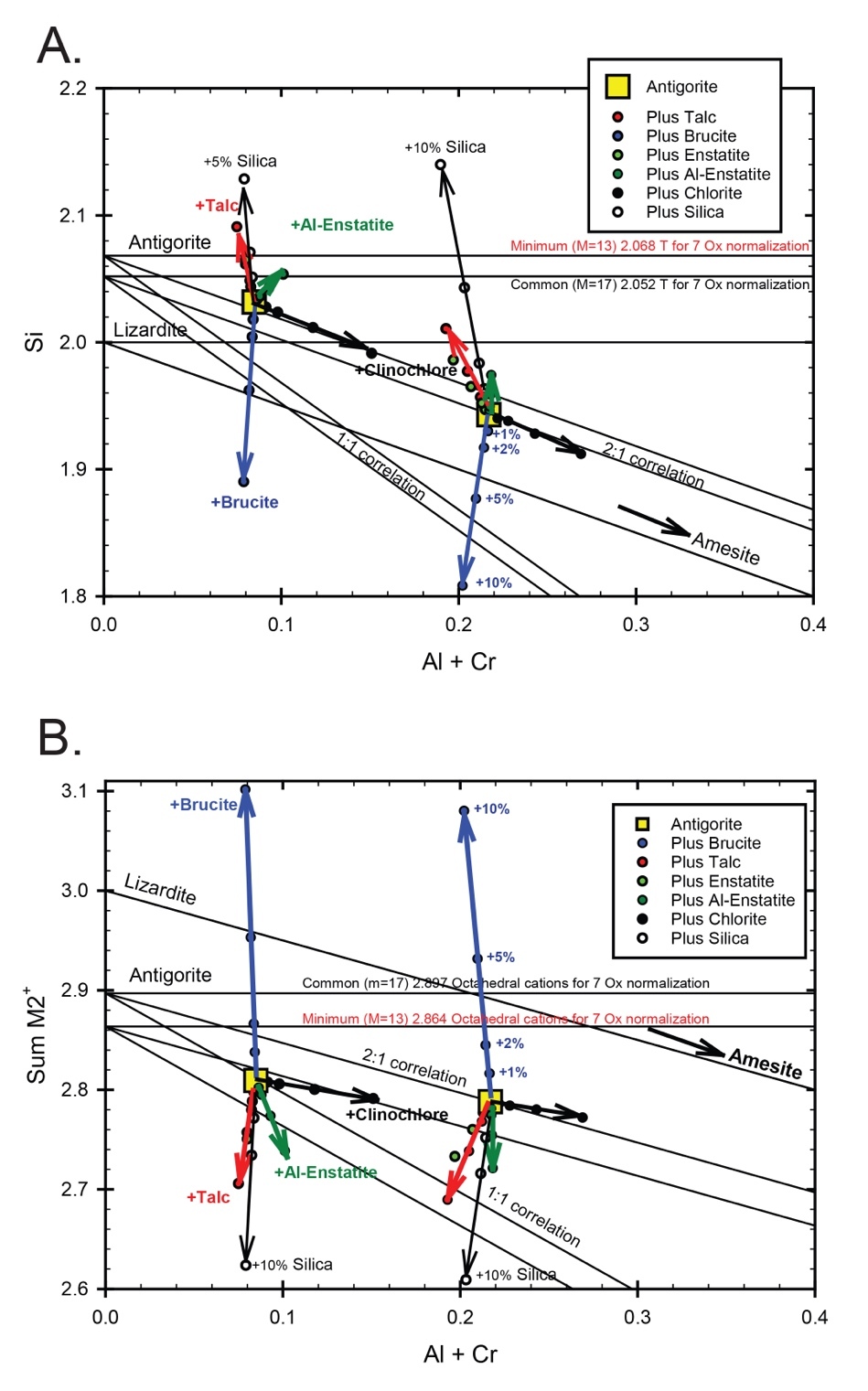
This is equivalent to a solid solution between Lz and amesite [Mg2Al(SiAl)O5(OH)4]. In plots of Si cations versus Al+Cr, Atg analyses should lie on lines extrapolating to Si ≈ 2.052 for m=17 or 2.068 for m=13 at Al+Cr = 0. For comparable plots of ΣM2+ = (Mg+Fe2++Mn+Ni+Co) versus Al+Cr, ΣM2+ ≈ 2.897 for m=17 and 2.864 for m=13. On the other hand, Lz analyses should lie on lines extrapolating to either Si ≈ 2 or ΣM2+ ≈ 3 at Al+Cr=0. The trends for a Tschermak’s exchange should lie on a line defining Si versus 0.5*(Al+Cr) (as half the Al+Cr should be replacing ΣM2+) or 2:1 (see fig. 5); a 1:1 line is shown for comparison. Analyses falling dramatically off these trends represent “contamination” by components like talc (super silicic), silica, or brucite (super magnesian) as contamination (nuggets) or interlayers as was interpreted by Veblen and Buseck (1979). These “contamination” trends are demonstrated in figure 5 including enstatite and clinochlore as possibilities and is discussed below.
Antigorite compositions from several antigoritites, typically from thin or polished section traverses, are plotted in figure 6 for two serpentinites together with an X-ray diffractogram to demonstrate variations that can be ascribed to serpentine alone versus intergrowths or contamination by another phase. Three versions are presented: i) total calculated tetrahedral cations [= Si + 0.5*(Al+Cr)] versus ΣM2+ cations minus Mg, so a sort-of reciprocal of Mg#*, which is an indicator of the serpentine’s precursor phase/protolith and the general scatter in composition; ii) Si versus Al+Cr, which can distinguish Atg from Lz and assess Tschermak’s exchange plus the presence of intergrowths or contamination; and iii) ΣM2+ versus Al+Cr, which should be a reciprocal assessment to plot ii. These plot types are also used in figures 5 and 7. So, in figure 6 for MVJ87-6-2, an NMM Atg serpentinite, figure 6A shows mostly compositions consistent with Atg in terms of T-site occupancy with a few analyses scattering to higher Fe+Mn+Ni+Co. Figure 6B & 6C show all but one of the analyses are well-behaved as Atg and, thus, maybe only one of the analyses higher in Fe+Mn+Ni is possibly the result of contamination. In support, the diffractogram that shows only Atg is given in figure 6D. In the case of MVE04-9-1, a SMM Atg serpentinite also plotted in figure 6, there is evidence of relatively constant Fe+Mn+Ni+Co (~0.25) and both excess Si off the Tschermak’s trend away from a common composition with (Al+Cr) ≈ 0.09 as well as considerable Si deficiency (Mg enrichment) away from the same common composition. Something like 10 wt% magnesite or 5 wt% brucite is required, which is not evident in the XRD data.
Representative compositions, along with diffractograms, from several Lz serpentinites are plotted in figure 7, in the same manner as for Atg serpentinites in figure 6. The noteworthy characteristics of the Lz/Ctl compositions is the commonly elevated Si content compared to standard stoichiometry, where the end member has 2 Si cations per 7 oxygens. Nonetheless, most of the analyses in each plot follow a Tschermak’s exchange trend, although displaced from the line aligning with Lz. Examples from all three tectonic settings are presented: A) ophiolite — MVE12-30-4, Cebadilla, BVP; B) an ophiolitic sliver in the NMM — MVE15-9-2, Quebrada Los Pozos near Saltan; and C) an ophiolitic sliver in the SMM — MVE15-17-4, Portreo Carillo. MVE12-30-4 was studied for boron isotopes by Martin et al. (2016), and its plots (fig. 7A) include adding either talc or silica to a nominal Lz analysis to guestimate the contamination. Opal, which would not be detected by XRD, is the likely source of excess silica. MVE15-9-2 and MVE15-17-4 were likewise reported on in Martin et al. (2023). Both show excess Si for being Lz but generally follow a 2:1 trend in the Si vs. (Al+Cr) plots. For plots of ΣM2+ versus (Al+Cr) (particularly plot Biii) there are clear departures from a Tschermak’s trend (2:1 relationship between ΣM2+ and [Al+Cr]) toward a 1:1 trend. Other than a fortuitous combination of excess silica balanced by a Tschermak’s exchange, it would require a component free of the summed M2+ cations, considering Ca does not solve the problem. Something like pyrophyllite [Al2Si4O10(OH)2] is needed to move compositions in that direction, for which there is no evidence let alone being unlikely in serpentinites. We are at a loss to explain this feature.
5.2. Spinels
Spinel group minerals include both the rarer primary chromian spinel of depleted mantle peridotites (e.g., MVE06-14-2 SMM), with evolved chromian spinel to magnesiochromites from ophiolite serpentinized harzburgites (MVE12-39-1 BVP or MVE10-16-3 JdP) to chromites in more fully reacted serpentinite or in podiform chromite (MVE04-25-2 or MVJ84-15.5-1 NMM) being more common. Magnetite, the product of serpentinization is the most abundant spinel phase in the Lz serpentinites, whereas in antigoritites it is less abundant to the point of being scarce, as discussed later. Representative analyses are presented in table 2.
5.3. Pyroxenes
Both orthopyroxene and clinopyroxene are found in serpentinites, either relics of the peridotite protoliths or secondary diopside, which is a byproduct of alteration and serpentinization. Augites can contain up to ~8 wt% Al2O3, suggesting high-T equilibration. Orthopyroxene exists as lamellae in exsolved relict primary grains with clinopyroxene. Representative analyses are presented in table 3.
Coexisting Cpx and Opx permit an estimation of the temperature and possibly pressure at which the pyroxenes coexisted, presumably in the protolith prior to serpentinization (table 4). Calculations were carried out using the Excel file “RiMG069_Ch03_two-pyroxene_P-T.xls” published by Putirka (2008). Estimates are similar in the range of 850 to 1000°C for the SMM rocks (no Opx found in NMM rocks) but more scatter in pressure from 5 to 7 kbar. These pressures are very low even for the chilled lithospheric mantle wedge at these temperatures, and the temperatures are at the low end of the calibration of the two-pyroxene thermobarometer.
5.4. Olivine
Olivine is rarely preserved and, essentially, just in peridotites that were only partially serpentinized. All compositions are appropriate for peridotite, and there is no evidence of metamorphic olivine. In a few rocks from the mélanges, olivine coexists with Atg (see fig. 2E&F) with spear-shaped laths of Atg piercing Ol. In these cases, there is an exchange between the two phases in a reaction relationship, antigorite consuming olivine and pyroxene. The olivine becomes locally enriched in fayalite component due to the higher partitioning/preference of magnesium into the adjacent Atg, although the average Atg is more magnesian still. For example, in MVE06-14-2, the average value of Mg/(Mg+Fe+Mn+Ni+Co) (Mg#*) in olivine is 0.897, whereas adjacent to Atg the value is as low as 0.811 and the average value in Atg is 0.964 versus 0.941 adjacent to olivine. Analogously, in other rocks:
MVE06-14-3: Ol – 0.905 vs 0.842; Atg – 0.952 vs 0.950.
MVE06-14-5: Ol – 0.913 vs 0.850; Atg – 0.966 vs 0.960
It should be noted that the Atg adjacent to Ol has a lower value of Mg#* than the average matrix Atg, indicating that Fe has been consumed by formation of magnetite. Although this represents frozen reaction progress, interpreting the Kd Ol/Atg for Mg#* at the boundary (~0.88) may be close to “equilibrium;” the value from the average compositions of Ol and Atg does not represent equilibrium. Representative analyses of olivine are presented in table 5.
5.5. Garnet
A pair of rocks from the SMM where Lws-eclogites are also exhumed (MVE02-15-14, MVE02-15-15) contain small grains of andradite mixed in shear bands with magnetite and diopside. Low Si apfu (<6/24ox) suggests perhaps 1 to 2 percent hydrogarnet content. In an ophiolitic serpentinite from the BVPU (MVJ84-20-4) small red grains of a potentially decomposed uvarovite-like composition were found mixed with a calcsilicate phase (wollastonite or xonotlite?). Analyses are presented in table 6.
5.6. Other minerals
Mn-ilmenite, talc, and chlorite are not uncommon, and brucite has been verified in only a single sample. All chlorite is clinochlore with values of Mg# between 91 and 95 and Si/(Al+Si) between 0.59 and 0.72 in Atg serpentinites and 96 and 0.57, respectively, in one Lz serpentinite. Clinochlore is present mostly in not-fully serpentinized peridotite adjacent to relict pyroxene. Carbonates as phenoblasts or in fractures between serpentine volumes include magnesite, dolomite, and ankerite; calcite is secondary in veins and fractures. Silica, primarily as opal, is found in cavities and fractures, mainly in Lz serpentinites. Results on sulfides shows pentlandite, mackinawite, heazlewoodite and millerite, which suggests low-T reequilibration and possible influence from late-stage fluids. Rare awaruite and Cu-awaruite are present. See table S1 for the distribution in studied rocks.
6. Discussion
6.1. Serpentinite protoliths
Textural interpretation of serpentinite protoliths is possible for relatively few of the antigoritites due to shear deformation destroying protolith textures, but harzburgites (bastite plus relics of mesh texture olivine replacement) appear to be dominant. The lizardite-dominant serpentinites, being only locally sheared, are easier to interpret as harzburgites. However, a detailed trace element whole rock analysis is beyond the scope of this work and will be presented elsewhere. The compositions of spinels measured in this study do provide some further inference about protolith. Various plots involving XCr-XMg (Dick & Bullen, 1984), Cr# vs TiO2 (Pearce et al., 2000) and TiO2 vs #Fe3+ (Dare et al., 2009) point to the most Mg-Al spinel-rich compositions representing partial mantle melt residue (e.g., DMM, value of Workman & Hart, 2005) as in abyssal peridotite, with evolution towards chromite (enriched also in ferric iron) by a possible combination of further reactions with melts in the mantle wedge and subsequent hydrous interaction (alteration and serpentinization) to drive compositions toward ferrit-chromite and magnetite in figure 8. As is consistent for harzburgite being the common protolith, preserved spinels plot near the value for primitive mantle and DMM and trend toward residue from melt residue before spreading into areas of melt-peridotite reaction and/or serpentinization.
6.2. Compositional zoning and non-stoichiometric serpentine compositions
An obvious aspect of examining microprobe analyses of serpentine from Guatemala is the compositional heterogeneity and departures from nominally stoichiometric compositions, even when including Tschermak’s exchanges. Zoning within a rock is most noteworthy with the difference between replacement of olivine versus bastite, because of higher Al and Cr content in serpentinized bastite versus higher Ni in serpentinized olivine (see table 1). The compositional trends following Tschermak’s exchanges show that diffusion or fluid transport of Al and Cr toward homogenization is not accomplished in any of these rocks. Mg# [100*Mg/(Mg+Fe2+); recall all Fe is treated as Fe2+] values appear to be closer among analyses with the greatest Al+Cr variance, but Fe3+ has not been measured or estimated, which will affect this value.
Attention to Fe3+ is considerable in the study of serpentinites because of the redox reactions that include formation of magnetite plus H2 and implications for fO2, fS2, and interactions with carbon-bearing species (cf. Andreani et al., 2013; Debret et al., 2014, 2015; Eberhard, Plümper, et al., 2023; Reynard et al., 2022). Evans et al. (2012) pointed out that Fe3+ can balance deficiency in the T site by balancing the Tschermak’s exchange with Al+Cr and suggested an average value of ~0.011 apfu or 0.83 wt% Fe2O3 in studied Atg, although the Mössbauer estimates of Fe3+ were generally lower. However, Evans et al. (2012) focused on prograde Atg formation from Lz/Ctl and, perhaps Ol, rather than direct hydration of a peridotite in a mantle-wedge setting. The possibility of formation of octahedral vacancies by oxidation of Fe2+ has been argued by Wicks and Plant (1979), Evans (2008), and Eberhard, Frost et al. (2023) with two Fe2+ ions oxidized to Fe3+ ions balanced by an octahedral vacancy; however, determining whether this occurs in our samples is beyond the scope of our data and this paper. As most T sites are calculated to be fully occupied with Si + Al (assuming trivalent cation balancing will leave Cr in the octahedral site) in well-behaved analyses, Fe3+ content must be small. For serpentine analyses that are subsilicic and relatively iron-rich, converting some assumed ferrous iron to ferric iron can move compositions to stoichiometry. An example is given in the following section.
With respect to non-stoichiometric compositions, not detecting a second phase by microdiffraction nor noteworthy departures from serpentine diffraction patterns suggests irregular interlayering of supersilicic (talc-like) or supermagnesian (brucite-like?) sheets in serpentine. Of course, this depends to some degree on the sensitivity of microdiffraction to detection of secondary phases and polytypism in the serpentine minerals that makes identifications problematic. Veblen and Buseck (1979) describe interlayers of talc in serpentine minerals but without XRD or compositional data to compare. Beyond these interlayers or some sort of nano inclusions, ferric iron can still be invoked for subsilicic serpentine stoichiometry.
6.3. Where are the magnetite and brucite?
Although phase abundance analyses were not carried out on the bulk serpentinites, initial use of REE magnets demonstrated that some antigorite-dominant serpentinites were conspicuously lacking in magnetite – magnets did not stick to some rocks, and opaques (on surfaces or in sections) in these were rare if not absent. This feature was most common in the rocks for which any evidence of protolith texture was absent, rather stress-oriented texture was dominant (e.g., Brownlee et al., 2013). This is not the case with some antigorite-dominant serpentinites that preserve much protolithic texture or with most Lz/Ctl serpentinites, which always contain Mag. Consequently, a quantitative assessment was made of opaques in thin sections of antigorite-dominant serpentinites (see table 7) using ImageJ as described above.
A critical aspect of peridotite hydration to form serpentine involves model redox reactions that create magnetite as well as brucite (e.g., Frost & Beard, 2007; Wicks & Whittaker, 1977). Magnetite formation results in part because serpentine preferentially partitions Mg relative to Fe2+ compared to olivine or orthopyroxene. Excess iron derived mainly from olivine must either form an iron-rich brucite (amakinite component – Fe2+(OH)2) or magnetite, via redox reactions involving reactant oxygen or product H2 (or CH4 if a carbon source is present). Most commonly, magnetite is observed in serpentinites.
The simplified reaction to form serpentine from olivine is:
2 Mg2SiO4Olivine+3 H2Ofluid=Mg3Si2O5(OH)4serpentine+Mg(OH)2brucite
But more realistically, peridotitic olivine contains iron (ignoring lesser iron in serpentine), so
34(Mg1.8Fe0.2SiO4)+(139.43)H2O=Mg48Si34O85(OH)62+13.2 Mg(OH)2+(6.83)Fe3O4+(6.83)H2
Consequently, magnetite is expected and hydrogen gas emission has been reported (e.g., Barnes et al., 1978; Wood, 1972). The near absence of magnetite in serpentinite could be attributed to ferric iron entering the serpentines (Frost, 1985). O’Hanley and Dyar (1993) determined roughly equal amounts of ferric and ferrous iron were incorporated in Lz and Atg of many Cassiar serpentinites (British Columbia, Canada). Some contain little magnetite, and all have relatively low total iron in serpentine: generally, less than 3 wt% FeO* and Mg# from 97.1 to 98.7 in Lz, whereas in Atg, Mg# is 96.0 to 96.8 except in one case, 91.3, in which Mag and Lz were replaced by Atg near a thrust fault. How does this compare with the samples studied here for explaining low magnetite?
Lizardite-dominant serpentinites from Guatemala generally contain Mag but also demonstrate a greater range of Mg# than recorded by the workers cited above: one has Mg# = 89 (MVE12-38-1—serpentinized bastite), but most range from 92 to 97.2. Ferric iron was not measured in these serpentines; however, converting Fe2+ to Fe3+ in the octahedral site should be balanced by moving an equivalent amount of octahedral Al to the T site, as pointed out by Evans et al. (2012) and Eberhard, Frost et al. (2023). For compositions that are not supersilicic (which are few), ferric iron can be estimated to allow a full T site occupancy. As an example, for the serpentinized bastite in MVE12-38-1, adding about 0.05 Fe3+ (apfu) brings the T and octahedral cations close to stoichiometry, but it does not work for the matrix Lz because it yields excess T site occupancy.
Antigorite-dominant serpentinite serpentines show a more distributed range of Mg# from 89.1 to 97.7 that is correlated positively with the presence/abundance of magnetite/opaque (table 7, fig. 9). The lowest values occur in serpentinites that can be semitranslucent and have low percentages of opaques (~<1%; see table 7). For these rocks with the lowest values of Mg# for which we also have whole-rock (WR) compositions, the Mg#s of serpentine and WR are roughly equal, thus consistent with the lack of magnetite. All the WR Mg#s only range from 89 to 91.7 with one exception at 93.1, a rock that has a wide secondary fibrous Atg vein (MVE04-18-3). If, as it appears, that Mg# of the protolith is basically preserved, what is the meaning of the variation in magnetite content and Atg compositions? The first question is whether there is another mineral competitor for Fe3+? Chlorite is rare, particularly in the rocks with the least amount of Mag, and all measured compositions are clinochlore with very low Fe, as noted in Section 5.6. Second, what might be the ferric iron content of the iron-rich Atg? Perhaps the Atg incorporates the ferric iron produced that would otherwise crystallize as magnetite.
As noted above, ferric iron can be estimated from the stoichiometry of Atg analyses that have deficient Si but otherwise do not look odd (such as with excess Mg). For MVE03-20-3 with an average Mg# = 89.6 (WR value of 89.7), about 0.5 Fe3+ apfu can be converted from “measured” Fe2+ to fill deficiency in the T site or about 17% of the total iron (compare with Evans, 2010). The model reaction by Frost and Beard (2007) transforms the iron in olivine (Fo90) completely into magnetite, with 66% Fe3+ (or Fe3+/FeT); however, the serpentine contains no Fe in the model calculation. Using the measured Mg# of Atg in contact with Fo90 of about 95 (see above), then the production of magnetite would be reduced, with the value of Fe3+/FeT reduced to 43%. This amount of ferric iron is still far greater than the amount in Atg predicted from stoichiometry and is large enough that magnetite should be conspicuous. Moreover, the roughly equivalent WR Mg# and Atg Mg# argues for virtually no magnetite. It would appear either the magnetite forming reaction never occurred or the Atg reequilibrated with the WR composition with no currently evident redox reaction preserved. This situation among the antigoritites is variable, perhaps reflecting in some cases, conditions, or process-related phenomena variably experienced by these mantle mélange rocks.
An alternative explanation for the lack of magnetite in some serpentinites was described by Klein et al. (2014) and Evans (2010). In the former, Fe3+ is shown by Mössbauer analysis to be incorporated into serpentine; however, these seafloor serpentines are probably Lz (identifications not reported) and, thus, inappropriate here. Evans (2010) argued that formation of Atg at higher T (~400+°C) in the mantle wedge will not produce Mag (or much Mag) because of the enhanced reactivity, diffusivity, and expanded Fe2+ solid solution range for Atg at higher T. Higher T of reaction will also lead to lower rock strength and, perhaps, explain the lack of preserved protolith texture resulting from deformational destruction of the pre-existing texture. Evaluation of temperature, such as via oxygen isotope exchange equilibria, has not been carried out. Consequently, the constraints are from the stability of Atg, the phase assemblage that does not include metamorphic olivine, and pressure estimates from study of jadeitites that formed in these serpentinite mélanges. The NMM assemblages range from about 0.6 to 1.2 GPa and the SMM ones from about 1.2 to 1.9 GPa (Harlow et al., 2011), which constrains maximum T for Atg stability at about 650°C (Bromiley & Pawley, 2003).
Compositions support an interpretation of reduced magnetite formation during serpentinization of antigoritites. Plots of Mg# for WR versus the average Mg# for Atg show reasonably well-defined groupings with little overlap (fig. 9). They are distinguished by distinct ranges of Atg Mg#: 89 to 92 and 94 to 98. There are outliers that are likely caused by talc or carbonate that speak to open system behavior with introduction of CO2, S, and clearly SiO2 (to explain the absence of brucite), but the tightness of the groupings suggests little change in whole-rock Mg and Fe.
The implication is that magnetite-poor antigoritites represent higher T and, as they are not abundant in our collection of mélange serpentinites, they represent a small fraction of the serpentinite mélange and, probably, a distinct sampling by the exhumation process. Consequently, we have plotted the magnetite-poor antigoritites separately from the others. At the scale of the map in figure 1, there does not appear to be any clustering of higher T serpentinites, perhaps suggesting higher and lower T antigoritite was distributed continuously along the faulted mantle wedge and, likewise, sampled randomly (or routinely) by the exhumation process.
What about brucite? Depending upon whether the hydration reactions include orthopyroxene as well as olivine, added silica is required to avoid brucite formation. For a harzburgite with equal olivine and orthopyroxene:
Mg2SiO4olivine+MgSiO3orthopyroxene+2 H2Ofluid=Mg3Si2O5(OH)4serpentine
no brucite is produced. For typical olivine-dominant peridotite protoliths, the lack of brucite must be explained. Malvoisin (2015) pointed out that more extensive fluid-rock interaction, particularly fluid introduction of silica will remove brucite from serpentinites, and, likewise, Klein et al. (2020) noted brucite dissolution in oceanic serpentinites with essentially complete serpentinization. Frost (1985) argued that silica in serpentinizing fluid is responsible for lack of brucite in ophiolitic Lz serpentinites. Extensive fluid-rock interaction and introduction of silica (and carbonate) is common in the rocks studied here, supporting an hypothesis that brucite dissolved or reacted out. The absence of brucite in all the serpentinites but one studied here requires either all the protoliths were harzburgites with balanced abundances of olivine and orthopyroxene (unlikely) or addition of silica or carbonate to the olivine-dominant protoliths.
6.4. Coexisting Mélange and Ophiolite Serpentinites:
A critical aspect of this study is the significance of Atg serpentinites in the two mélange belts and the occasional Lz serpentinites in fault slivers hosted in mélanges, particularly for the NMM. The Atg serpentinites predominate in the NMM and SMM. Their integrity as larger blocks (as noted above), both in terms of size of block, and coherence of the rocks, is common, particularly in the NMM. Antigoritite in smaller fault slivers in the mélanges tends to be less coherent and more heavily sheared but still dominant. As Atg is the higher temperature serpentinite mineral and is associated in the mélanges with HP–LT blocks (eclogite, garnet amphibolite, jadeitite: Flores et al., 2013; Harlow et al., 2011, 2015) formed in a subduction channel, the likely source of the Atg serpentinites is the hydrated margin of the overlying mantle wedge. This interpretation is supported by the δ11B systematics of both serpentine and minerals in blocks from the NMM and SMM (Martin et al., 2016, 2020) that show low δ11B for crystallization products of subduction channel fluid.
The presence of fault slivers of Lz serpentinite within the mélanges needs an explanation. These bodies have all the characteristics of ophiolite, including containing rodingite veins. Particularly, rodingites bearing vesuvianite ((Ca,Na)19(Al,Mg,Fe)13(SiO4)10(Si2O7)4(OH,F,O)10) or prehnite (Ca2Al2Si3O10(OH)2) are not found in high-T (eclogite-grade?) metamorphosed rodingites (meta-rodingites, e.g., Bach & Klein, 2009; Laborda-López et al., 2018). Consequently, these rodingites support both the typical greenschist-like conditions as well as the sub-seafloor context of an ophiolitic association. All the Lz serpentinites in the mélanges exist as smaller (km-scale) fault slivers aligned parallel to the major faults of the MFS (see figure 1). The juxtaposition of mantle-wedge antigoritites with ophiolitic Lz serpentinites implies tectonic imbrication of both deep-originated slices with shallow slices of oceanic lithosphere. The location of these mélanges along a plate boundary fault system which has evolved to strike-slip motion must be important. Other comparable associations that include two types of serpentinite plus jadeitite exist in the Sierra del Convento of eastern Cuba (Cárdenas-Párraga et al., 2012, 2017, 2021; García-Casco et al., 2009) and the Río San Juan Complex of northern Dominican Republic (Hertwig et al., 2016, 2021; Schertl et al., 2012, 2015).
Serpentinite from north of the MFS commonly contains magnesite which suggests higher levels of CO2 in the serpentinizing fluids and may result from the distinct ~70 Ma event recorded in the entrained tectonic metabasites. The samples from the south more commonly contain Lz with Atg, which is consistent with colder, and perhaps even wetter, conditions that produced lawsonite eclogites during the ~120 Ma event. The combination of interpreted original lithologies, mineralogies, and mineral chemistries are most consistent with origin from a complex mantle-wedge peridotite assemblage.
7. Conclusion
Several observations and conclusions result from this study of extensively collected serpentinites in the Guatemala suture zone. First, antigoritites dominate the mélange fragments in the NMM and SMM compared to lizardite (Lz/Ctl) serpentinites from the ophiolite bodies (primarily the Baja Verapaz Ultramafic). However, small slices of Lz serpentinite are found in the more fragmented sections of what have previously interpreted as mélange slivers and sometimes contain rodingite veins/dikes. These latter bodies are indistinguishable from ophiolite and suggest some tectonic juxtaposition of the serpentinites of different origin in the mélanges strung along the boundary of the GSZ.
Compositions of the serpentines indicate heterogeneity on a grain size scale and in some rocks variance from ideal stoichiometry, tending towards “contamination” by talc, silica, or brucite, yet the diffraction results do not indicate such contamination is by inclusions but rather by interlayering within the crystals’ structures. These features argue for a lack of equilibrium within serpentinites and a remarkable flexibility in serpentine internal structure. Likewise, the “contamination” aspect argues for open system behavior with respect to addition silica for the talc-like or silica-rich substitutions.
Similarly, brucite is essentially absent from all serpentinites, inconsistent with model reactions for hydration of ultramafic protoliths. The simplest interpretation is that the missing brucite has reacted to form either more serpentine, carbonate (magnesite or dolomite) or talc by the addition of CO2 or SiO2 by the serpentinizing fluid. Some brucite component may also exist as intracrystal layering described in the previous paragraph.
In particular, for antigorite-dominant serpentinites, a minority contain little to no magnetite, inconsistent with commonly accepted serpentinite-forming reaction that does produce magnetite. The simplest interpretation is that of Evans (2010), that such Mag-poor/Mag-absent antigoritites formed at relatively high T (≥400 °C to <650 °C) at which the hindrances for forming Fe-richer Atg are overcome, contrary to the interpretation of Klein et al. (2014) for seafloor serpentinization producing lower amounts of Mag at lower T. Alternatively, a complex process of H2 production from a hydrous fluid to produce Mag and then a process to reduce Fe3+ (change of conditions or more H2?) to permit formation of a more Fe-rich antigorite. Nevertheless, the presence of both Mag-bearing and Mag-absent serpentinite requires sampling and preserving different paragenetic regimes within the suprasubduction zone mantle from whence the serpentinites formed.
Consequently, the Guatemala serpentinites represent a very dynamic assemblage and an entry point for the understanding of serpentinite formation.
Acknowledgments
We mourn the passing of our collaborator, colleague, and friend Sorena S. Sorensen. She was an important contributor to this work and others, and the geoscience community; her absence is a great loss.
We sincerely appreciate the valuable comments and suggestions from our reviewers, José Alberto Padrón-Navarta and an anonymous reviewer, and associate editor, which have significantly improved the quality of this manuscript. We want to thank Carlos Gonzales for field assistance; Eric Sahm, Nancy Price, and Jamie Newman for analytical and sample processing assistance; Federico Caro for access to XRD instrumentation at the Metropolitan Museum of Art; and John Cleary for sharing geological observations from mapping observations on the deposits near Carrizal Grande. This research was supported by grants from the US National Science Foundation, EAR 0309320, and 1119403 awarded to G.E.H., and EAR 0309116 to VBS, EAR 1951166 to C.M, EAR 2150618 to K.E.F., and in part by the start-up budget of C.M. at the University of North Carolina at Charlotte. In addition, partial field support was provided by the American Museum of Natural History.
Author Contributions
George Harlow is the fundamental author of the text and interpretation, particularly for mineral-specific information. Kennet Flores contributed detailed structural, tectonic, and field interpretation, plus constructing the geological map. Céline Martin has contributed with field and data collecting and a new overview supported by boron isotopic research (e.g., Martin et al., 2020). Virginia Sisson and Sorena Sorensen have been long-term partners in research on the GSZ, both with field work, sample analysis and overall interpretation.
Data and Supplementary Information
Data related to the specimens studied in the manuscript are available from the senior author and will be registered through SESAR and deposited in EarthChem, both part of the IEDA2 Data Facility, funded by the National Science Foundation (NSF).
https://doi.org/10.60520/IEDA/113704 (Harlow et al., 2025)
Editor: C. Page Chamberlain, Associate Editor: Jay Ague

--_a)_antigorite_--_mve06-7-8_(d-blo.jpeg)
_of_both_antigorite_and_lizardite_in.jpeg)

)_for_representative_antig.jpeg)
_serpentini.jpeg)
_xcr_--_xmg_and_ycr-xmg_plots_after_dick_and_bul.jpeg)
_opaque_(a_proxy_for_mag)_abundance_and_b)_whole_rock_mg___among.jpeg)
--_a)_antigorite_--_mve06-7-8_(d-blo.jpeg)
_of_both_antigorite_and_lizardite_in.jpeg)
)_for_representative_antig.jpeg)
_serpentini.jpeg)
_xcr_--_xmg_and_ycr-xmg_plots_after_dick_and_bul.jpeg)
_opaque_(a_proxy_for_mag)_abundance_and_b)_whole_rock_mg___among.jpeg)